Steve McDaniel and Jonathan D. Hurt, Reactive Surfaces Ltd., LLP., Austin, Texas04.09.18
What if, because of paint, man could finally answer the age-old question: Are We Alone?
It is often said amongst materials scientists that the very last thing considered by equipment design engineers is the materials from which the equipment will be constructed. While that’s a wee bit of an exaggeration, rarely are materials the first thing considered in design of space probes, including sophisticated equipment whose mission is directed to searching for microbial life forms on solar system bodies other than Earth. That approach is about to change. The changes to coatings necessitated by the extreme requirements of life-seeking space missions are some of the same changes generally needed for many planet Earth coatings applications.
Changes to the design of life-seeking probes will come about as a result of coating advances which are capable of reducing the probability of forward and backward microbial contamination to a statistical likelihood of zero. To understand how important this is, consider the National Aeronautics and Space Administration (NASA) estimates that 100s of thousands of living bacterial spores hitched a ride on the Curiosity rover that is travelling around in Gale Crater on Mars today.1 Because the experiments used to detect microbiological signatures of Earth-like microbial life are extremely sensitive, no mission-critical surface can harbor Earth-derived microbes or viruses, or biological macromolecules such as cellular debris, lipid membranes, enzymes, or nucleic acids that contain genetic information. The extreme requirements for elimination of any and all false positive results can be met by self-sterilizing coatings that allow no Earth-derived microbe, virus or free nucleic acids to forward contaminate an exoplanet target where there is likely to be liquid water and, thus, potential extraterrestrial life. False positive elimination becomes especially critical where such detection is based upon detecting exceedingly rare microbes or informational molecules. If return of an exoplanet sample to Earth is to be conducted, the same rigorous specifications for coatings that continually self-decontaminate and self-sterilize will be enforced to prevent unintentional release of backward contamination.
These advanced coatings being developed for spacecraft will find a “1 g” application that will likely have far-reaching implications in the areas of human health. For instance, such coatings will likely play a critical role in reducing the environmental development of antimicrobial resistance and its rapid-spread through microbial populations by the transfer of DNA (and, in a more limited fashion, RNA). Such genetic transfers are routinely seen from cells that can survive a challenge by an antibiotic to other antibiotic sensitive cells using standard processes of gene transfer including cell replication and cellular exchange of nuclear materials. In more limited instances, cells are able to pick up intact environmental nucleic acids from surfaces with which they come into contact – by encountering naked strands of intact DNA/RNA or by encountering environmentally-stable bacteriophages (viruses that attack and take over the genetic and metabolic machinery of bacteria).2
Sterilization of the Penetrator Probe and Inclusion of Self-Sterilizing Coatings
To ensure the integrity of life detection experiments on exoplanets and prevent interplanetary biological contamination, a probe delivered from earth needs to be sterilized to kill contaminating organisms and destroy detectable terrestrial biomolecules such as proteins, nucleic acids, carbohydrates, and lipids. To maintain sterility after whatever initial process is used (e.g., heat, irradiation, sterilizing gases, clean room techniques), virtually all the surfaces of the probe will be coated with a self-sterilizing coating that contains non-toxic biomolecule additives (bio-additives) that kill and/or sequester terrestrial life.
For example, a bio-additive known as an enzyme catalyzes chemical reactions that detrimentally alter a bio-molecule for which the enzyme has a specific binding affinity, such as a protease enzyme that binds a polypeptide to hydrolytically cleave that polypeptide into smaller, non-functional molecules. In another example, biological lipids encapsulate living cells and certain viruses, and these lipid structures are integral to maintaining the living function of a cell. A very small biomolecule known as a peptide, which is an oligomer of amino acid monomers, may interact with these biological lipids to produce holes in the encapsulating material that can render a cell lifeless. Some biomolecule additives may be selected for inclusion in a coating to sequester microbes rather than immediately kill them. An antibody that binds a specific type of biomolecule commonly found on the surface of microorganisms may be incorporated in a coating to bind the microorganisms or biomolecules to the coating’s surface.
Additionally, a coating may be formulated with certain peptides that chelate trace metals needed in the function of certain enzymes involved in biological metabolism, thus producing a biocidal or biostatic effect by inhibiting those key enzymes.
Combinations of Bio-additives for Synergistic Self-Sterilizing Effects
Combining coating bio-additives with different modes of action for killing terrestrial microorganisms would be the most effective way to produce a self-sterilizing coating in space exploration applications. Combinations of bio-additives previously used in a coating include a biomolecule degrading enzyme, lysozyme, and a peptide capable of producing holes in encapsulating lipids, ProteCoat®.3 Lysozyme was selected as it catalyzes the cleavage of certain sugar derivatives that act as crosslinkers in biological polymers that make up cell walls in bacteria. The cell walls of bacteria are part of their outer surface, and breakage of the cell wall’s integrity will lead to loss of critical internal material needed for maintaining living function and entry of undesired material such as excess water. Surface lipids of bacteria possess a negatively charged chemical structure attached to a hydrophobic carbon chain. Many millions of these lipids form a bi-layer that encapsulates the internal components of bacterial cells. The ProteCoat peptide is made of alternating positively charged and uncharged amino acid monomers in its oligomer chain. The resulting molecule possesses a positively charged face and a hydrophobic face that will associate with bacterial surface lipids. A plurality of ProteCoat molecules binding to the lipid bilayer of bacteria will reorganize the lipids to produce holes in the encapsulating bi-layer, allowing detrimental loss of internal material and entry of undesirable external material into the cell.
In Gram-positive bacteria, the cell wall susceptible to lysozyme is at the surface of the microorganism with lipid bilayers susceptible to ProteCoat underneath, while the reverse configuration exists in Gram-negative bacteria. Combining the two bio-additives allows improved degradation of bacteria surfaces as one bio-additive would permeabilize the topmost layer of material and aid access of the other bio-additive to attack the next layer.
The effect of combining both these bio-additives has been evaluated against the Gram-positive microorganism Micrococcus lysodeikticus, and synergistic activity observed.3 Technical paper coupons (e.g., up to 5 x 40 mm) treated with a coating comprised of one or both of the bio-additives at concentrations up to 2% lysozyme and/or 0.5% ProteCoat were contacted with a solution of M. lysodeikticus in a cell lysis assay. A 100 mm2 coupon size, for example, exhibited 44% lysis, whereas the dual bio-additive paper exhibited 93%. The observed/expected ratio was 2.1 (93/44+0) which is indicative of synergistic cell lysis activity.3
Reduction of Terrestrial Contamination via Nuclease Coatings
The blueprint for each living organism on Earth is encoded in nucleic acids and assays for detecting nucleic acids will likely be included in a penetrator probe’s scientific equipment package. As extraterrestrial microbes may be few in number due to the harsher environments in the outer solar system, highly sensitive nucleic acid detection assays will likely be used. For instance, polymerase chain reaction is sensitive enough to use a single strand of extraterrestrial nucleic acid and biochemically reproduce it millions of times to create a readily detectable signal. But conversely, this means that a single strand of contaminating terrestrial nucleic acid can produce a false positive signal.
A second and important issue is that contamination of an exoplanet with terrestrial life does not require a whole living microbe from Earth to land on a new home world and begin dividing and growing. A possible way to permanently alter the genetics of an exoplanet’s endogenous microbial life would be for a nucleic acid encoded gene from Earth to survive on a surface of a penetrator probe, and then contact and become incorporated into an exoplanet microbe’s genetic makeup.
We have shown that a self-sterilizing coating having one or more enzymes known as nucleases can be used to degrade any contaminating nucleic acids and mitigate these concerns. On Earth, life uses two nucleic acids, the well-known deoxyribonucleic acid (DNA), as well as lesser known ribonucleic acid (RNA), which is often used by viruses. Both DNA and RNA are generally linear tetrapolymers, and nuclease enzymes known as exonucleases hydrolytically cleave these types of polymers at their ends (e.g., Phosphodiesterase I), while endonuclease enzymes cleave the polymer chain at internal locations (e.g., DNase I, EcoRI). The more degraded a nucleic acid is, the less likely it is that such a nucleic acid polymer will successfully transform the genome of an indigenous extraterrestrial microbe.
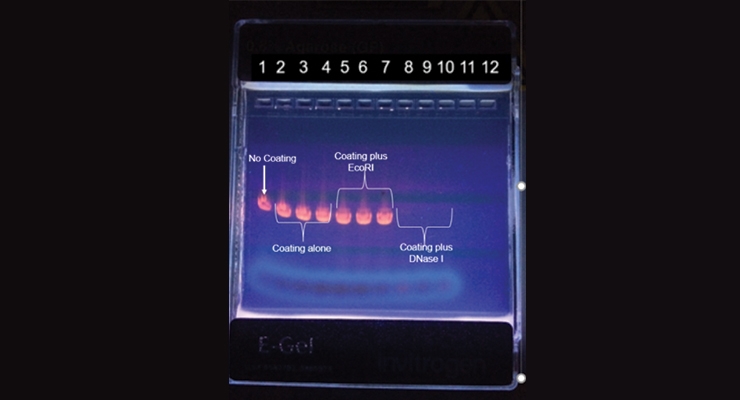
In order to test the ability of such bio-additives to produce a DNA-free surface, an acrylic latex paint was formulated with an exonuclease, Phosphodiesterase I (from Eastern Diamondback rattlesnake), capable of degrading both DNA and RNA polymer strands. Cured free films of the acrylic latex were placed in a liquid assay with a nucleic acid ester cleavable by the enzyme, thymidine 5-monophosphate p-nitrophenyl ester, and demonstrated degradation of this substrate.3 In another study, Minwax coatings were mixed with either DNase I, an endonuclease that will non-specifically degrade a multi-thousand nucleotide unit-long DNA or RNA polymer into mono-, di- and tri-nucleotide oligomers; or an endonuclease, EcoRI, that will only cleave DNA at a very specific sequence effectively leaving little size change in the DNA molecule after cleavage. A DNA molecule of known size was contacted for 35 minutes at 20 °C in wells of a 96-well plate containing either a no-coating control, Minwax without any bio-additive, Minwax functionalized with the EcoRI endonuclease, or Minwax functionalized with DNase I. The contents of each reaction were placed in an agarose gel and the reaction products separated by size using electrophoresis.
The lanes of the agarose gel contains reaction products of DNA contacted with either no-coating (lane 1); Minwax without any bio-additive (lanes 2-4); Minwax functionalized with EcoRI endonuclease (lanes 5-7); or Minwax functionalized with DNase I (lanes 8-10). Lanes 11-12 had no samples added. The gel was stained with ethidium bromide, a dye that will bind to and fluoresce intact DNA molecules pink-orange when placed in ultraviolet light. No detectable DNA was seen in the gel lanes containing reaction products of DNA contacted with DNase I - functionalized Minwax, demonstrating that the coating efficiently degraded the DNA (Figure 1).
Thus, use of a nuclease with broad specificity for both types of terrestrial nucleic acids (DNA and RNA) is a viable solution to detection of potential false positives and cross-planet genetic contamination. Similarly, where there is concern that DNA possessing antimicrobial resistance factors may reside upon an at-risk surface (e.g., surgical suite equipment), and that such DNA might give rise to transfer of such resistance factors to non-resistant microbes, such nuclease-functionalized surface coatings will likely play a role in diminishing that threat.
The Possible Origin(s) and Locations of Life in Our Star System
Exoplanet microbial life may be biochemically similar to terrestrial life – at any rate, it is the sort of microbial life we know how to detect and analyze, ergo an exclusive focus on Earth-like microbial life is reasonable. One explanation of this may be because it has been shown that it is possible that microbial life within the solar system may be transplanted from one planet or moon to another within the solar system.4 The most likely transplantation mechanism is an impact of an asteroid or comet ejecting a hardy microorganism, such as bacteria living inside a rock, into space. Mars likely had a more life-favorable environment including an ocean that covered almost a third of the planet about 4.1 to 3.7 billion years ago, so intra-solar cross contamination between Earth and Mars could have resulted in both planets having genetically and biochemically similar microbial life. Of course, life may have independently arisen on more than one planetary body (i.e., a second or separate genesis event). In that case, exoplanet microbial life could have clearly different nucleic acid and biomolecule structures from Earth-based organisms. Alternatively, microbial life from outside the solar system, such as bacteria contained in extra-solar comet(s) or asteroids (e.g., the extra-solar asteroid Oumaumau recently detected in the solar system5), could have deposited evolutionarily-related microbes to various planets or moons, in a process referred to as panspermia.
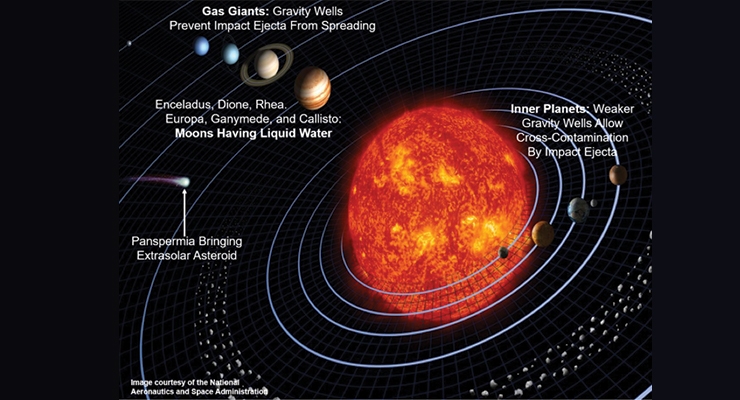
In any case, planetary bodies that had or presently have liquid water would be the targets for life-detecting probes. The list for NASA’s search for life within the solar system continues to grow, and presently includes Saturn’s moons Enceladus, Dione, and Rhea, Jupiter’s moons Europa, Ganymede, and Callisto, Mars, the asteroid Ceres, and of course, Earth (Figure 2).
Large impacts from asteroids or comets are capable of ejecting surface material containing microbial life far into space from smaller planets such Earth and Mars that may contaminate another planet or moon. Impact ejecta from a moon orbiting a gas giant would likely be trapped by the gas giant’s gravity well and prevented from spreading to another planet, though it might spread to another nearby moon. Life brought from outside the solar system may be deposited anywhere in the solar system, and later spread by other impacts.
Planetary Life-Detection Probe Design
Robotic probe engineers can now aim directly at and confidently analyze even the most sensitive of potential life-bearing ecosystems and niches (aquifers, ice caps, subterranean caves and karsts, etc.) that heretofore have been strictly hands-off. Of particular concern would be the ease of any terrestrial microbe to rapidly infect an entire extraterrestrial ecosystem after the probe contacts exoplanet aquifers or sub-surface oceans produced by gravitational tidal heating and/or radioisotope decay, such as the sub-surface ocean found on Europa (Figure 3).
Recently, NASA invited materials scientists (including scientists from Reactive Surfaces) and other investigators in related disciplines to discuss and help design approaches and equipment to accomplish detection of extraterrestrial microbial life.6 One configuration for such a scientific package that was submitted by our team was a penetrating probe in the shape of a dart or lance that would ballistically impact the surface of a celestial body after being dropped from a spacecraft in orbit.7 Deeply-penetrating military bomb technology (“bunker-buster” bombs) can be used in life detection probes to achieve a desired depth in a target regolith. It is thought that for a planet such as Mars, life may exist below the surface to avoid sterilizing irradiation from space, such as ultraviolet light penetrating the thin Martian atmosphere.
Furthermore, liquid water capable of sustaining microbial life would likely exist below the surface as surface moisture would evaporate rapidly due to the low atmospheric pressure. By punching a hole in the surface, the probe can readily retrieve sub-surface dirt samples at layers more conducive to a microbial ecosystem.
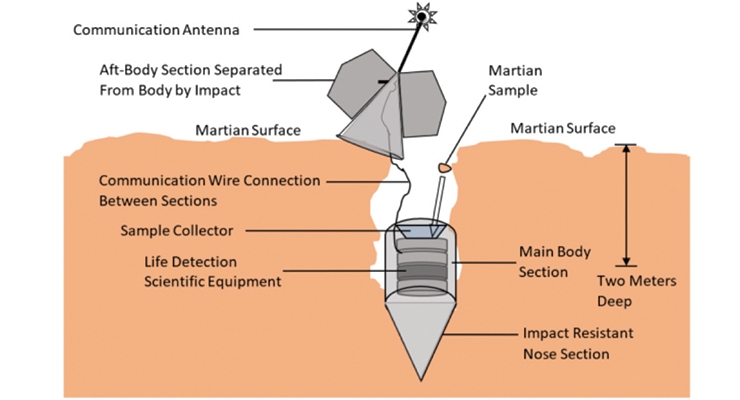
A hardened nose section would allow the probe and scientific equipment package to achieve sub-surface depth during impact (Figure 4). In some configurations of the probe design, the impact with the surface opens the probe to allow ease of exoplanet material collection. The life detection scientific equipment contained in the probe’s main body section will then conduct one or more experiments to evaluate whether life is present in the samples. The aft-body section will remain upon or near the surface to allow radio communication of the life detection results to orbiting spacecraft (not shown), and may be connected to the life detection equipment by communication wire as surface material might interfere with wireless communication with a probe that becomes buried after impact. In all cases, all surfaces of each component of the penetrator probe will be coated with self-sterilizing, self-cleaning coatings in order to eliminate forward contamination by hitch-hiker microbes or their genetic material.
On Mars, ice sheets of greater than 100 meters thick have been recently found at the mid-latitudes of the planet.8 These sheets are covered by one to two meters of surface material, and would be prime targets for a ballistic probe to impact and achieve a sub-surface depth to sample this ice for traces of life.
Additional Uses for Self-Sterilizing Coatings on Earth
Self-sterilizing coatings would have further utility in additional space applications and on Earth. For example, use of traditional chemical in-film preservatives in the enclosed spaces of manned space missions for prolonged missions may have detrimental effects on the health of astronauts. Self-sterilizing coatings would go far in reducing these issues, and could also be applied on Earth in similar closed environments, where people live and work in enclosed spaces (e.g., buildings, aircraft, ships, hospitals). Hospitals in particular could benefit from self-sterilizing coatings. Nuclease-containing coatings may reduce the development of antibiotic resistant pathogenic strains in environments such as hospitals by destroying such nucleic acids on surfaces before a pathogen has a chance to utilize them.
Paint was certainly one of mankind’s (and it appears his relative Homo neanderthalensis relatives9) earliest innovations. From the earliest paintings, it is clear that humans were contemplating deep philosophical issues, including where man fit in the cosmic puzzle. If he now uses paint wisely, he may just be able to solve some of these puzzles and, in the process, make his “cave” a safer place to live.
References
Benardini, J.N.III.; La Duc, M.T.; Beaudet, R.A.; Koukol, R. Implementing Planetary Protection Measures on the Mars Science Laboratory. Astrobiology. 2014, 14, 27-32.
Levy, S.B.; Marshall, B. Antibacterial resistance worldwide: causes, challenges and responses. Nature Medicine. 2004, 10, S122-S129.
C. S. McDaniel, Anti-fouling Paints and Coatings, U.S. Patent Application 14/097,128, July 9, 2015.
McKay, D.S.; Gibson Jr., E.K.; Thomas-Keprta, K.L.; Vali, H.; Romanek, C.S.; Clemett, S.J.; Chillier, X.D.F.; Maechling, C.R.; Zare, R.N. Search for Past Life on Mars: Possible Relic Biogenic Activity in Martian Meteorite ALH84001”. Science. 1996, 273, 924–930.
Fitzsimmons, A.; Snodgrass, C.; Rozitis, B.; Yang, B.; Hyland, M.; Seccull, T.; Bannister, M.T.; Fraser, W.C.; Jedicke, R.; Lacerda, P. Spectroscopy and thermal modelling of the first interstellar object 1I/2017 U1 ‘Oumuamua. Nature Astronomy. 2018, 2, 133-137.
Seeking the Tricorder, Workshop on Advanced Technologies for Life Detection, NASA Ames, Moffett Blvd, Mountain View, CA, May 19 - 20, 2017.
C. S. McDaniel, Life Seeking Exoplanet Penetrator, U.S. Patent Application 14/979,980, unpublished work.
Dundas, C.M.; Bramson, A.M.; Ojha, L.; Wray, J.J.; Mellon, M.T.; Byrne, S.; McEwen, A.S.; Putzig, N.E.; Viola, D.; Sutton, S.; Clark, E.; Holt, J.W. Exposed subsurface ice sheets in the Martian mid-latitudes. Science. 2018, 349, 199-201.
Appenzeller, T. Old masters: The earliest known cave paintings fuel arguments about whether Neanderthals were the mental equals of modern humans. Nature, 2013, 497, 302–304.
Contact information: smcdaniel@reactivesurfaces.com, 512-472-8282
i This article introduces concepts for control of microbes on at-risk surfaces that will be expanded upon in a three-part series in Coatings World entitled “The Molecular Biology of Coatings” that will discuss: replacing traditional in-process, in-can, and in-film biocides with species-specific bio-based biocides; extracting extremely rare nucleic acid polymers from liquid coatings and raw materials in order to detect very early microbial contamination; using molecular biology to counteract biofilms created by microbial consortia.
It is often said amongst materials scientists that the very last thing considered by equipment design engineers is the materials from which the equipment will be constructed. While that’s a wee bit of an exaggeration, rarely are materials the first thing considered in design of space probes, including sophisticated equipment whose mission is directed to searching for microbial life forms on solar system bodies other than Earth. That approach is about to change. The changes to coatings necessitated by the extreme requirements of life-seeking space missions are some of the same changes generally needed for many planet Earth coatings applications.
Changes to the design of life-seeking probes will come about as a result of coating advances which are capable of reducing the probability of forward and backward microbial contamination to a statistical likelihood of zero. To understand how important this is, consider the National Aeronautics and Space Administration (NASA) estimates that 100s of thousands of living bacterial spores hitched a ride on the Curiosity rover that is travelling around in Gale Crater on Mars today.1 Because the experiments used to detect microbiological signatures of Earth-like microbial life are extremely sensitive, no mission-critical surface can harbor Earth-derived microbes or viruses, or biological macromolecules such as cellular debris, lipid membranes, enzymes, or nucleic acids that contain genetic information. The extreme requirements for elimination of any and all false positive results can be met by self-sterilizing coatings that allow no Earth-derived microbe, virus or free nucleic acids to forward contaminate an exoplanet target where there is likely to be liquid water and, thus, potential extraterrestrial life. False positive elimination becomes especially critical where such detection is based upon detecting exceedingly rare microbes or informational molecules. If return of an exoplanet sample to Earth is to be conducted, the same rigorous specifications for coatings that continually self-decontaminate and self-sterilize will be enforced to prevent unintentional release of backward contamination.
These advanced coatings being developed for spacecraft will find a “1 g” application that will likely have far-reaching implications in the areas of human health. For instance, such coatings will likely play a critical role in reducing the environmental development of antimicrobial resistance and its rapid-spread through microbial populations by the transfer of DNA (and, in a more limited fashion, RNA). Such genetic transfers are routinely seen from cells that can survive a challenge by an antibiotic to other antibiotic sensitive cells using standard processes of gene transfer including cell replication and cellular exchange of nuclear materials. In more limited instances, cells are able to pick up intact environmental nucleic acids from surfaces with which they come into contact – by encountering naked strands of intact DNA/RNA or by encountering environmentally-stable bacteriophages (viruses that attack and take over the genetic and metabolic machinery of bacteria).2
Sterilization of the Penetrator Probe and Inclusion of Self-Sterilizing Coatings
To ensure the integrity of life detection experiments on exoplanets and prevent interplanetary biological contamination, a probe delivered from earth needs to be sterilized to kill contaminating organisms and destroy detectable terrestrial biomolecules such as proteins, nucleic acids, carbohydrates, and lipids. To maintain sterility after whatever initial process is used (e.g., heat, irradiation, sterilizing gases, clean room techniques), virtually all the surfaces of the probe will be coated with a self-sterilizing coating that contains non-toxic biomolecule additives (bio-additives) that kill and/or sequester terrestrial life.
For example, a bio-additive known as an enzyme catalyzes chemical reactions that detrimentally alter a bio-molecule for which the enzyme has a specific binding affinity, such as a protease enzyme that binds a polypeptide to hydrolytically cleave that polypeptide into smaller, non-functional molecules. In another example, biological lipids encapsulate living cells and certain viruses, and these lipid structures are integral to maintaining the living function of a cell. A very small biomolecule known as a peptide, which is an oligomer of amino acid monomers, may interact with these biological lipids to produce holes in the encapsulating material that can render a cell lifeless. Some biomolecule additives may be selected for inclusion in a coating to sequester microbes rather than immediately kill them. An antibody that binds a specific type of biomolecule commonly found on the surface of microorganisms may be incorporated in a coating to bind the microorganisms or biomolecules to the coating’s surface.
Additionally, a coating may be formulated with certain peptides that chelate trace metals needed in the function of certain enzymes involved in biological metabolism, thus producing a biocidal or biostatic effect by inhibiting those key enzymes.
Combinations of Bio-additives for Synergistic Self-Sterilizing Effects
Combining coating bio-additives with different modes of action for killing terrestrial microorganisms would be the most effective way to produce a self-sterilizing coating in space exploration applications. Combinations of bio-additives previously used in a coating include a biomolecule degrading enzyme, lysozyme, and a peptide capable of producing holes in encapsulating lipids, ProteCoat®.3 Lysozyme was selected as it catalyzes the cleavage of certain sugar derivatives that act as crosslinkers in biological polymers that make up cell walls in bacteria. The cell walls of bacteria are part of their outer surface, and breakage of the cell wall’s integrity will lead to loss of critical internal material needed for maintaining living function and entry of undesired material such as excess water. Surface lipids of bacteria possess a negatively charged chemical structure attached to a hydrophobic carbon chain. Many millions of these lipids form a bi-layer that encapsulates the internal components of bacterial cells. The ProteCoat peptide is made of alternating positively charged and uncharged amino acid monomers in its oligomer chain. The resulting molecule possesses a positively charged face and a hydrophobic face that will associate with bacterial surface lipids. A plurality of ProteCoat molecules binding to the lipid bilayer of bacteria will reorganize the lipids to produce holes in the encapsulating bi-layer, allowing detrimental loss of internal material and entry of undesirable external material into the cell.
In Gram-positive bacteria, the cell wall susceptible to lysozyme is at the surface of the microorganism with lipid bilayers susceptible to ProteCoat underneath, while the reverse configuration exists in Gram-negative bacteria. Combining the two bio-additives allows improved degradation of bacteria surfaces as one bio-additive would permeabilize the topmost layer of material and aid access of the other bio-additive to attack the next layer.
The effect of combining both these bio-additives has been evaluated against the Gram-positive microorganism Micrococcus lysodeikticus, and synergistic activity observed.3 Technical paper coupons (e.g., up to 5 x 40 mm) treated with a coating comprised of one or both of the bio-additives at concentrations up to 2% lysozyme and/or 0.5% ProteCoat were contacted with a solution of M. lysodeikticus in a cell lysis assay. A 100 mm2 coupon size, for example, exhibited 44% lysis, whereas the dual bio-additive paper exhibited 93%. The observed/expected ratio was 2.1 (93/44+0) which is indicative of synergistic cell lysis activity.3
Reduction of Terrestrial Contamination via Nuclease Coatings
The blueprint for each living organism on Earth is encoded in nucleic acids and assays for detecting nucleic acids will likely be included in a penetrator probe’s scientific equipment package. As extraterrestrial microbes may be few in number due to the harsher environments in the outer solar system, highly sensitive nucleic acid detection assays will likely be used. For instance, polymerase chain reaction is sensitive enough to use a single strand of extraterrestrial nucleic acid and biochemically reproduce it millions of times to create a readily detectable signal. But conversely, this means that a single strand of contaminating terrestrial nucleic acid can produce a false positive signal.
A second and important issue is that contamination of an exoplanet with terrestrial life does not require a whole living microbe from Earth to land on a new home world and begin dividing and growing. A possible way to permanently alter the genetics of an exoplanet’s endogenous microbial life would be for a nucleic acid encoded gene from Earth to survive on a surface of a penetrator probe, and then contact and become incorporated into an exoplanet microbe’s genetic makeup.
We have shown that a self-sterilizing coating having one or more enzymes known as nucleases can be used to degrade any contaminating nucleic acids and mitigate these concerns. On Earth, life uses two nucleic acids, the well-known deoxyribonucleic acid (DNA), as well as lesser known ribonucleic acid (RNA), which is often used by viruses. Both DNA and RNA are generally linear tetrapolymers, and nuclease enzymes known as exonucleases hydrolytically cleave these types of polymers at their ends (e.g., Phosphodiesterase I), while endonuclease enzymes cleave the polymer chain at internal locations (e.g., DNase I, EcoRI). The more degraded a nucleic acid is, the less likely it is that such a nucleic acid polymer will successfully transform the genome of an indigenous extraterrestrial microbe.
In order to test the ability of such bio-additives to produce a DNA-free surface, an acrylic latex paint was formulated with an exonuclease, Phosphodiesterase I (from Eastern Diamondback rattlesnake), capable of degrading both DNA and RNA polymer strands. Cured free films of the acrylic latex were placed in a liquid assay with a nucleic acid ester cleavable by the enzyme, thymidine 5-monophosphate p-nitrophenyl ester, and demonstrated degradation of this substrate.3 In another study, Minwax coatings were mixed with either DNase I, an endonuclease that will non-specifically degrade a multi-thousand nucleotide unit-long DNA or RNA polymer into mono-, di- and tri-nucleotide oligomers; or an endonuclease, EcoRI, that will only cleave DNA at a very specific sequence effectively leaving little size change in the DNA molecule after cleavage. A DNA molecule of known size was contacted for 35 minutes at 20 °C in wells of a 96-well plate containing either a no-coating control, Minwax without any bio-additive, Minwax functionalized with the EcoRI endonuclease, or Minwax functionalized with DNase I. The contents of each reaction were placed in an agarose gel and the reaction products separated by size using electrophoresis.
The lanes of the agarose gel contains reaction products of DNA contacted with either no-coating (lane 1); Minwax without any bio-additive (lanes 2-4); Minwax functionalized with EcoRI endonuclease (lanes 5-7); or Minwax functionalized with DNase I (lanes 8-10). Lanes 11-12 had no samples added. The gel was stained with ethidium bromide, a dye that will bind to and fluoresce intact DNA molecules pink-orange when placed in ultraviolet light. No detectable DNA was seen in the gel lanes containing reaction products of DNA contacted with DNase I - functionalized Minwax, demonstrating that the coating efficiently degraded the DNA (Figure 1).
Thus, use of a nuclease with broad specificity for both types of terrestrial nucleic acids (DNA and RNA) is a viable solution to detection of potential false positives and cross-planet genetic contamination. Similarly, where there is concern that DNA possessing antimicrobial resistance factors may reside upon an at-risk surface (e.g., surgical suite equipment), and that such DNA might give rise to transfer of such resistance factors to non-resistant microbes, such nuclease-functionalized surface coatings will likely play a role in diminishing that threat.
The Possible Origin(s) and Locations of Life in Our Star System
Exoplanet microbial life may be biochemically similar to terrestrial life – at any rate, it is the sort of microbial life we know how to detect and analyze, ergo an exclusive focus on Earth-like microbial life is reasonable. One explanation of this may be because it has been shown that it is possible that microbial life within the solar system may be transplanted from one planet or moon to another within the solar system.4 The most likely transplantation mechanism is an impact of an asteroid or comet ejecting a hardy microorganism, such as bacteria living inside a rock, into space. Mars likely had a more life-favorable environment including an ocean that covered almost a third of the planet about 4.1 to 3.7 billion years ago, so intra-solar cross contamination between Earth and Mars could have resulted in both planets having genetically and biochemically similar microbial life. Of course, life may have independently arisen on more than one planetary body (i.e., a second or separate genesis event). In that case, exoplanet microbial life could have clearly different nucleic acid and biomolecule structures from Earth-based organisms. Alternatively, microbial life from outside the solar system, such as bacteria contained in extra-solar comet(s) or asteroids (e.g., the extra-solar asteroid Oumaumau recently detected in the solar system5), could have deposited evolutionarily-related microbes to various planets or moons, in a process referred to as panspermia.
In any case, planetary bodies that had or presently have liquid water would be the targets for life-detecting probes. The list for NASA’s search for life within the solar system continues to grow, and presently includes Saturn’s moons Enceladus, Dione, and Rhea, Jupiter’s moons Europa, Ganymede, and Callisto, Mars, the asteroid Ceres, and of course, Earth (Figure 2).
Large impacts from asteroids or comets are capable of ejecting surface material containing microbial life far into space from smaller planets such Earth and Mars that may contaminate another planet or moon. Impact ejecta from a moon orbiting a gas giant would likely be trapped by the gas giant’s gravity well and prevented from spreading to another planet, though it might spread to another nearby moon. Life brought from outside the solar system may be deposited anywhere in the solar system, and later spread by other impacts.
Planetary Life-Detection Probe Design
Robotic probe engineers can now aim directly at and confidently analyze even the most sensitive of potential life-bearing ecosystems and niches (aquifers, ice caps, subterranean caves and karsts, etc.) that heretofore have been strictly hands-off. Of particular concern would be the ease of any terrestrial microbe to rapidly infect an entire extraterrestrial ecosystem after the probe contacts exoplanet aquifers or sub-surface oceans produced by gravitational tidal heating and/or radioisotope decay, such as the sub-surface ocean found on Europa (Figure 3).
Recently, NASA invited materials scientists (including scientists from Reactive Surfaces) and other investigators in related disciplines to discuss and help design approaches and equipment to accomplish detection of extraterrestrial microbial life.6 One configuration for such a scientific package that was submitted by our team was a penetrating probe in the shape of a dart or lance that would ballistically impact the surface of a celestial body after being dropped from a spacecraft in orbit.7 Deeply-penetrating military bomb technology (“bunker-buster” bombs) can be used in life detection probes to achieve a desired depth in a target regolith. It is thought that for a planet such as Mars, life may exist below the surface to avoid sterilizing irradiation from space, such as ultraviolet light penetrating the thin Martian atmosphere.
Furthermore, liquid water capable of sustaining microbial life would likely exist below the surface as surface moisture would evaporate rapidly due to the low atmospheric pressure. By punching a hole in the surface, the probe can readily retrieve sub-surface dirt samples at layers more conducive to a microbial ecosystem.
A hardened nose section would allow the probe and scientific equipment package to achieve sub-surface depth during impact (Figure 4). In some configurations of the probe design, the impact with the surface opens the probe to allow ease of exoplanet material collection. The life detection scientific equipment contained in the probe’s main body section will then conduct one or more experiments to evaluate whether life is present in the samples. The aft-body section will remain upon or near the surface to allow radio communication of the life detection results to orbiting spacecraft (not shown), and may be connected to the life detection equipment by communication wire as surface material might interfere with wireless communication with a probe that becomes buried after impact. In all cases, all surfaces of each component of the penetrator probe will be coated with self-sterilizing, self-cleaning coatings in order to eliminate forward contamination by hitch-hiker microbes or their genetic material.
On Mars, ice sheets of greater than 100 meters thick have been recently found at the mid-latitudes of the planet.8 These sheets are covered by one to two meters of surface material, and would be prime targets for a ballistic probe to impact and achieve a sub-surface depth to sample this ice for traces of life.
Additional Uses for Self-Sterilizing Coatings on Earth
Self-sterilizing coatings would have further utility in additional space applications and on Earth. For example, use of traditional chemical in-film preservatives in the enclosed spaces of manned space missions for prolonged missions may have detrimental effects on the health of astronauts. Self-sterilizing coatings would go far in reducing these issues, and could also be applied on Earth in similar closed environments, where people live and work in enclosed spaces (e.g., buildings, aircraft, ships, hospitals). Hospitals in particular could benefit from self-sterilizing coatings. Nuclease-containing coatings may reduce the development of antibiotic resistant pathogenic strains in environments such as hospitals by destroying such nucleic acids on surfaces before a pathogen has a chance to utilize them.
Paint was certainly one of mankind’s (and it appears his relative Homo neanderthalensis relatives9) earliest innovations. From the earliest paintings, it is clear that humans were contemplating deep philosophical issues, including where man fit in the cosmic puzzle. If he now uses paint wisely, he may just be able to solve some of these puzzles and, in the process, make his “cave” a safer place to live.
References
Benardini, J.N.III.; La Duc, M.T.; Beaudet, R.A.; Koukol, R. Implementing Planetary Protection Measures on the Mars Science Laboratory. Astrobiology. 2014, 14, 27-32.
Levy, S.B.; Marshall, B. Antibacterial resistance worldwide: causes, challenges and responses. Nature Medicine. 2004, 10, S122-S129.
C. S. McDaniel, Anti-fouling Paints and Coatings, U.S. Patent Application 14/097,128, July 9, 2015.
McKay, D.S.; Gibson Jr., E.K.; Thomas-Keprta, K.L.; Vali, H.; Romanek, C.S.; Clemett, S.J.; Chillier, X.D.F.; Maechling, C.R.; Zare, R.N. Search for Past Life on Mars: Possible Relic Biogenic Activity in Martian Meteorite ALH84001”. Science. 1996, 273, 924–930.
Fitzsimmons, A.; Snodgrass, C.; Rozitis, B.; Yang, B.; Hyland, M.; Seccull, T.; Bannister, M.T.; Fraser, W.C.; Jedicke, R.; Lacerda, P. Spectroscopy and thermal modelling of the first interstellar object 1I/2017 U1 ‘Oumuamua. Nature Astronomy. 2018, 2, 133-137.
Seeking the Tricorder, Workshop on Advanced Technologies for Life Detection, NASA Ames, Moffett Blvd, Mountain View, CA, May 19 - 20, 2017.
C. S. McDaniel, Life Seeking Exoplanet Penetrator, U.S. Patent Application 14/979,980, unpublished work.
Dundas, C.M.; Bramson, A.M.; Ojha, L.; Wray, J.J.; Mellon, M.T.; Byrne, S.; McEwen, A.S.; Putzig, N.E.; Viola, D.; Sutton, S.; Clark, E.; Holt, J.W. Exposed subsurface ice sheets in the Martian mid-latitudes. Science. 2018, 349, 199-201.
Appenzeller, T. Old masters: The earliest known cave paintings fuel arguments about whether Neanderthals were the mental equals of modern humans. Nature, 2013, 497, 302–304.
Contact information: smcdaniel@reactivesurfaces.com, 512-472-8282
i This article introduces concepts for control of microbes on at-risk surfaces that will be expanded upon in a three-part series in Coatings World entitled “The Molecular Biology of Coatings” that will discuss: replacing traditional in-process, in-can, and in-film biocides with species-specific bio-based biocides; extracting extremely rare nucleic acid polymers from liquid coatings and raw materials in order to detect very early microbial contamination; using molecular biology to counteract biofilms created by microbial consortia.