Matthew Besterci, Cecilia M. McGough and Wilson Nova-Ruiz, Lanxess Corporation07.05.18
Abstract
As the industrial market shifts toward new and increasingly water-based polymer dispersions, the need for biocides to protect the integrity of the finished product increases. When fouled, polymer dispersions may exhibit a number of undesirable side-effects such as viscosity reduction, separation, and production of gas or odor. The diversity and complexity of these new systems presents a challenge to biocide selection. In industrial applications of polymer in water-based systems (e.g., coatings, adhesives, and sealants), analytical testing of the system enables a formulator to maximize biocide efficacy, thus protecting product integrity. Proper analytical screening including assay determination via High Performance Liquid Chromatography (HPLC), redox potential, pH measurement and general raw material compatibility testing helps in the development of an effective microbial protection program to inhibit or control the growth of microorganisms that cause biodeterioration. This paper addresses some key areas where analytical testing can provide critical information to ensure the optimal protection of polymer dispersions.
Introduction
Water-based polymer dispersions are colloidal polymer particles dispersed in water.1
The size of the colloidal polymer particles is usually in the range of 1 nm to 1 µm. The average industrial water-based polymer dispersions contain 40-60 percent colloid polymer particles. Typical industrial polymer dispersions are usually free-flowing with varying physical properties (e.g., rheology) based on the monomers used. These properties provide the functional backbone of the industrial application formulations. Polymer dispersions are major building blocks in the formulation of coatings, adhesives, paper coatings and other industrial products. Manufacturing and the composition of these polymer dispersions have been well documented in previous industry papers.1, 2, 3, 4
In general, water-based polymer dispersions have a high water content and high nutrient level providing ideal conditions for microbial growth. Microorganisms and their metabolic by-products can contribute to the breakdown of the polymer dispersion. Once the polymer dispersion has been contaminated, the properties and functionalities are affected. The use of biocides is critical to control and inhibit the growth of microorganisms within water-based polymer dispersions.
Important Test Methods
In the development of a biocide program for a polymer dispersion system, analytical testing can provide valuable information. Some tests are simple, cheap, and easy to perform while others are complex and require expensive equipment to obtain valuable data. Proactive analytical screening for pH and redox potential can be done very quickly and will aid in the selection of a proper biocide for a given system. HPLC is an effective way to monitor concentration of a biocide active ingredient. It is a more expensive and complicated form of testing, but when done properly, it is a powerful tool for verification of biocide compatibility and stability.
pH
The pH is defined as the negative logarithm of the hydrogen ion concentration. The numerical value is a ratio of the [H+] and [OH-] concentration in a given substance. In pure water at 25oC, the concentrations of [H+] and [OH-] are equal giving a pH value of seven, or neutral. Values below seven are acidic indicating a greater concentration of hydrogen ions, and values above seven are basic or alkaline indicating a greater concentration of hydroxyl ions.
When determining pH, some important factors must be considered regarding the selection of proper analytical equipment and test protocols. Choosing the right electrode is vitally important and there are many options. Basically, a pH measurement involves the use of two electrodes, the pH sensor and the reference electrode. Both electrodes are built into a single probe that is directly submerged into the sample solution. The most common reference system used is the silver/silver chloride system which consists of a silver wire coated with silver chloride in an electrolyte solution of potassium chloride saturated with silver chloride. Another factor in selecting the proper pH probe is the type of junction on the electrode. The junction is the opening in the reference electrode that maintains contact with the test sample. The sleeve junction is the best choice for testing dispersions due to the large contact area which reduces potential clogging of the junction by contaminant matter. Other specialized probes can offer performance advantages in extreme chemistries or temperatures.
Calibration of the pH meter is very important and usually involves either two or three buffer solutions preferably in or near the range of the test sample. Standard buffer solutions for polymer dispersions are at pH values of four, seven, and ten. Temperature has an effect on pH. Either a temperature probe should be used in conjunction with the pH probe or an attempt should be made to keep the temperature of the standard buffer solutions and test samples constant. Proper and frequent calibration will ensure that the readings are as accurate as possible.6
Redox Potential
Standard reduction potential (i.e., redox potential) is a measurement of the tendency of a substance to be reduced. Reduction is the gain of electrons. Thus, a measurement of redox potential is determining the likelihood that a sample will gain electrons; this is represented by a numerical value in millivolts (mV). The standard hydrogen electrode (SHE) is the basis for comparison of results. The redox potential of the SHE at room temperature is known to be zero. All results from various electrodes can be calibrated to read as the values that would be obtained with the SHE by using a conversion table from the manufacturer of the standard. Positive values indicate that the solution has oxidizing power, and negative values indicate reductive strength.8
As with pH probes, there are various electrodes that have attributes that are beneficial for specific test conditions. There are also various electrolyte solutions based on the ionic strength of the test matrix. It is important that the proper electrode is selected based on the polymer dispersion formulation. The recommended test protocols should be followed to ensure accuracy of the test results. Calibration using the ORP standard should be done before each test period to verify that the reported results are valid and that the device is working properly.
High Performance Liquid Chromatography
HPLC is an effective chromatographic process for the detection and quantification of biocide active ingredients. There are multiple parts of the process that add up to a powerful analytical technique. HPLC itself refers to the process and equipment that takes a sample and separates the components so that they can be collected or detected by various means. A sample is dissolved in solvent and forced through a tightly packed column at high pressure to enable separations with high resolution. Typically, ultraviolet-visible light detectors are coupled with HPLC systems through the use of a flow cell. The development of an effective HPLC method can be a long and complicated process, but it is the best method to monitor the compatibility of a biocide with a given system.
Sample preparation is the first and most critical step in the HPLC process. Polymer dispersions are a complex matrix from which to extract biocides. This step can be tackled with a mixture of research and trials. Solubility data in solvents for biocides is easily obtained and can start as a basis for the extraction process. The goal is to extract the biocide from the sample while removing contaminants. A good extraction solvent will contain all of the eluents in the chromatography process so that any reactions of the sample resulting in precipitates will occur prior to injection into the HPLC. The mixture of components in the solvent can be varied to achieve certain reactions. For example, an extraction of biocide from a polymer dispersion might cause the latex to crash out of the solution. The sample can then be centrifuged, and the liquid supernatant taken from the vial, thus removing the solid contaminants. Prior to injection, the sample should be passed through a sub-micron filter to ensure that no solid particles clog the column. A freeze-thaw process could also cause precipitate formation in a polymer dispersion sample. Solubility data and the eluent system are good information from which to base an extraction scheme but, ultimately, trial and error may lead to the best results overall.
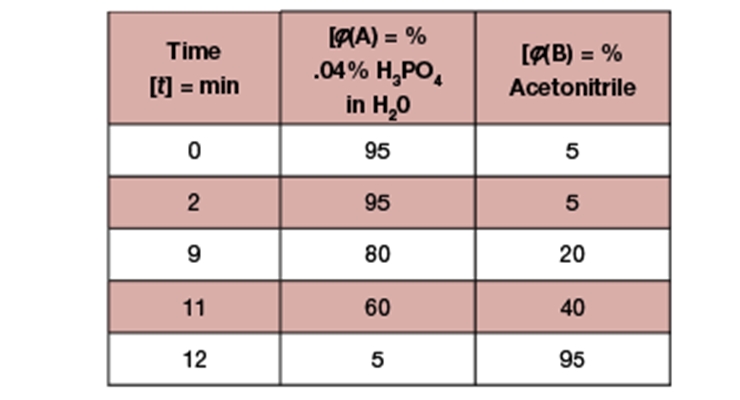
Once a clean sample is acquired, the next step of the process comes into focus. The chromatography process is the most important part of the method development and may require the most time. Modern HPLC systems use reversed-phase (RP) chromatography which is an inversion of normal-phase chromatography. RP chromatography uses a non-polar packing material in the column and has a mobile phase that starts out polar and is shifted to a more non-polar mobile phase as is necessary. Based on an affinity for the mobile phase over the stationary phase, different components will be retained in the column for different amounts of time. Isocratic elution involves a mobile phase that does not change composition. Gradient elution involves multiple eluents (aqueous and organic, polar and non-polar) that are mixed in different ratios by a program in the machine (Figure 1). This allows for high resolution and shorter run times.10
Choosing the right column, flow rate, eluent compositions, and gradient profile can be a difficult process if done from scratch. Luckily, there are some standard starting points for biocides that can significantly shorten the development process. Nearly all of the biocides for polymer dispersions work well with reversed-phase systems composed of C18-packed columns and some mixture of acetonitrile and water as the non-polar and polar eluents respectively. As is seen in Figure 1, a typical gradient profile starts with a higher percentage of the aqueous phase and gradually shifts toward nearly an organic phase. The polar components in the sample will elute early due to a lack of affinity for the stationary phase, and the non-polar components will elute as the mobile phase shifts toward a mostly organic composition. Many hours can be spent optimizing this system to fit various needs; speed can be prioritized over resolution or the other way around. There might not be such a thing as a bad HPLC method as long as the results can be validated and are reproducible.
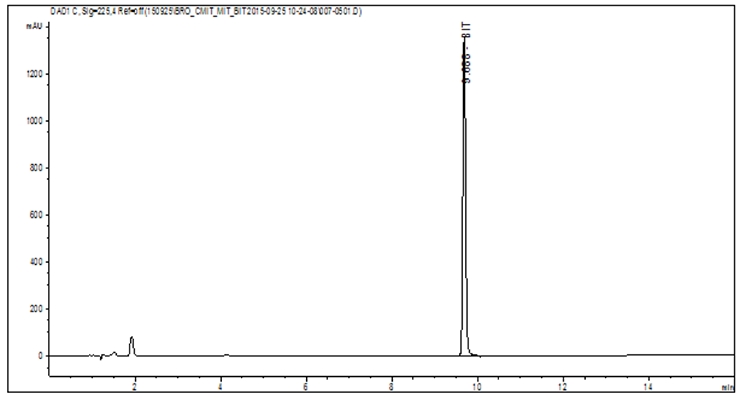
The final step of the HPLC process is the detection. If the wavelength of maximum absorption of the analyte is known, then this aspect of the test method is quite simple. If the wavelength of maximum detection is unknown for a certain analyte, and cannot be found in literature, then the process becomes more involved, and a more advanced detector might be required. Ultraviolet-Visible (UV-VIS) detection measures the absorption of wavelengths of light as they pass through a flow cell. As the components elute from the column, they pass through the flow cell absorbing light in the process. Absorbance is related to concentration. Therefore, quantification of the analyte can be achieved. The results are presented as a chromatogram, and determination of the area under the peak allows for concentration calculation (Figure 2).10
Only one wavelength can be measured using UV-VIS spectrometry so, if the proper wavelength is not known, this detection method is not ideal. A photodiode array detector (PDAD) operates similarly to a UV-VIS detector except that it can monitor a spectrum of wavelengths at once (Figure 3). Indeed, the PDAD can be used to determine the wavelength of maximum absorption of an analyte. Also, if there is interference from eluents or other components in the sample, the PDAD can be used to monitor the analyte at a wavelength where there is less interference.
Once all of the test method parameters have been adequately refined, it is important to validate that the method is accurate, precise, and reproducible. A typical analytical method validation for HPLC involves the use of standards with either multiple runs that can be averaged and/or a calibration curve with a linear fit line. One must first create a three-point calibration curve with a standard of known concentration and then test the calibration curve for accuracy, precision, reproducibility, and limit of detection. If the method/curve passes all of the tests, the method is validated, and there is assurance of the validity of the results.
Analytical Approach To Biocide Selection
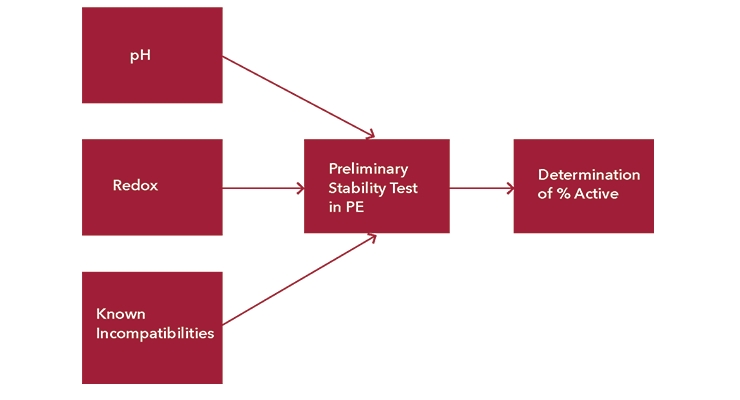
Effective microbial protection of a polymer dispersion requires an in-depth look into the chemistries of both the matrix and the biocides. Knowing your chemistries, you can develop a scheme to simplify the selection process. Figure 4 outlines a systematic approach to biocide selection using the analytical tools referenced in the previous section.
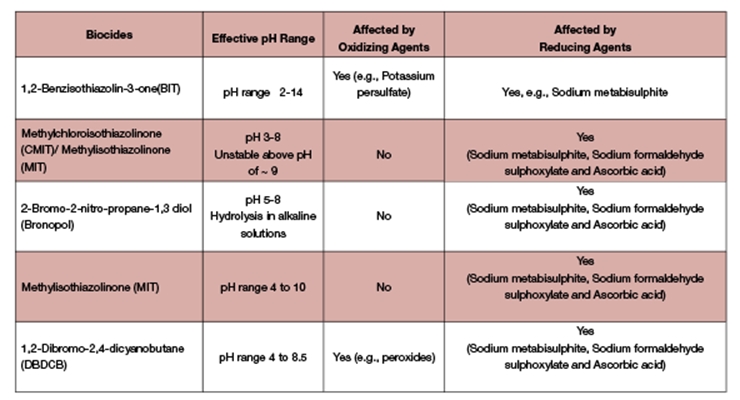
Initial screening includes a review of the pH and redox potential of the polymer dispersion. These conditions provide the basic criteria from which to select the appropriate and most stable biocide under given conditions. The pH of a typical polymer dispersion ranges between 3.5 and 10.0. Ethylene vinyl acetate (EVA) and polyvinyl acetate (PVA) are examples of acidic polymer dispersions, whereas styrene butadiene is an example of a basic polymer dispersion. Most biocides are stable in slightly acidic to neutral pH ranges, but there are limited options in alkaline environments. For example, BIT shows efficacy across a broad range including pH 8.0 and above where there are limited options. Biocide options for polymer dispersions below pH 8.0 include bronopol, CMIT/MIT, and DBDCB. See Table 1 for more detailed information regarding effective pH ranges for biocides.
Redox reagents are critical for the dispersion polymerization process. However, residual levels of initiators and/or terminators (i.e., free radicals) in the cooled latex can impact the performance of the biocides due to the highly reactive nature of those chemistries. CMIT/MIT is susceptible to attack from reducing agents present in the cooled polymer matrix, thereby hindering the performance of the biocide. As opposed to CMIT/MIT, BIT can be unstable in oxidative environments. Monitoring the redox potential of the system during the manufacturing process can predict potential incompatibilities and determine the best time to incorporate the proper biocide system into the process. Table 1 provides common redox chemistries that impact biocide stability.
The next stage in the biocide selection process is the trial and error phase. The results from the initial screening (i.e., pH and redox data) provide the basis for the trials.
Compatibility trials are run with potential biocide candidates and evaluated by visual assessment. Precipitates, coagulation, and color changes are indicators of an incompatibility between the polymer dispersion system and the biocide. Yellowing could indicate a breakdown of the biocide. Coagulation is an indication that the electrostatic surfactant(s) may have been affected by the biocide. Polyvalent-salt stabilized biocides have been known to alter the properties of polymer dispersion systems. Monovalent stabilized biocides may be used as an alternative.
A lack of visual cues does not mean that the biocide is present and effective. Additional testing is needed to confirm microbial protection. Measurement of the active biocide concentration is the last step in the analytical approach to biocide selection. Biocides are only effective when used in the proper dosage levels for a given matrix.
One of the most powerful analytical tools for biocide detection is HPLC. Even though method development for HPLC can be a time-consuming process, the results obtained are vitally important to ensure microbial protection. The procedure for developing an HPLC method for biocide determination is outlined in the previous section. Using this procedure and additional information from the biocide manufacturer can demystify and simplify the development process significantly.
Conclusions
An analytical approach to biocide selection can save time and money for the polymer dispersion manufacturer. Redox potential and pH are simple and effective ways to perform an initial screening of the preservation program of a polymer dispersion system. They can provide basic guidelines for biocide selection, but further testing should be performed. A visual assessment of a dispersion after biocide addition may show coagulation or precipitation, thus indicating a possible incompatibility, or it may show no sign at all. HPLC can provide certainty about compatibility even if no outwardly visible signs of issues are present. It also confirms that the appropriate biocide dosage levels are present in the system.
Once the proper biocide system has been selected, microbial challenge testing should be performed to verify the biocide effectiveness. A basic principle to follow is that if the biocide is unstable in the polymer dispersion system, then microbial control is compromised. Therefore, it is crucial to confirm that the biocide is stable in the polymer dispersion system to allow for proper microbial control.
Disclaimer
The manner in which you use and the purpose to which you put and utilize our products, technical assistance and information (whether verbal, written or by way of production evaluations), including any suggested formulations and recommendations are beyond our control. Therefore, it is imperative that you test our products, technical assistance and information to determine to your own satisfaction whether they are suitable for your intended uses and applications. This application-specific analysis must at least include testing to determine suitability from a technical as well as health, safety, and environmental standpoint. Such testing has not necessarily been done by us. Unless we otherwise agree in writing, all products are sold strictly pursuant to the terms of our standard conditions of sale. All information and technical assistance is given without warranty or guarantee and is subject to change without notice. It is expressly understood and agreed that you assume and hereby expressly release us from all liability, in tort, contract or otherwise, incurred in connection with the use of our products, technical assistance, and information. Any statement or recommendation not contained herein is unauthorized and shall not bind us.
Nothing herein shall be construed as a recommendation to use any product in conflict with patents covering any material or its use. No license is implied or in fact granted under the claims of any patent.
1 Anderson C.D.; Daniels E.S. Emulsion Polymerisation and Latex Applications, ort 160, ISSN: 0889-3144 Volume Number 4, 2003.
2 McGough, C. M.; Nova-Ruiz, W.; Weigenand, O. The Fundamental of Choosing A Biocide to Protect a Polymer Dispersion including Technical, Environmental and Regulatory Issues.
3 Urban D.; Takamura, K. Polymer Dispersions and Their Industrial Application, 2002 Wiley VCH, p.6
4 Chern, C.S., Emulsion Polymerization Mechanisms and Kinectics, Progress in Polymer Science, Vol 31(2006) p. 443-486.
5 Hach Company. (2010). What Is pH And How Is It Measured? A Technical Handbook for Industry. Hach Company.p.6.
6 Mettler-Toledo AG. (2007, 10). A Guide to pH Measurement - The Theory and Practice of Laboratory pH Applications. Switzerland: Mettler-Toledo AG. p. 5-15.
7 Mettler-Toledo AG. (2007, 10). A Guide to pH Measurement - The Theory and Practice of Laboratory pH Applications. Switzerland: Mettler-Toledo AG. p. 18-26.
8 Harris, D. C. (2007). Quantitative Chemical Analysis (Seventh Edition ed.). New York, NY: W.H. Freeman and Company. p. 270-279.
9 Thermo Fisher Scientific Inc. (2007). User Guide: Redox/ORP Electrodes. p.6
10 Harris, D. C. (2007). Quantitative Chemical Analysis (Seventh Edition ed.). New York, NY: W.H. Freeman and Company. p. 556-583.
11 Erstling, K. Lanxess Deustchland, Leverkusen, Germany. Internal Communication, 2015.
12 Gillatt, J.W. The Microbial Spoilage of Polymer Dispersions and its Prevention in Directory of Microbicides for the Protection of Materials, A Handbook Edited by Wilfired Paulus p. 219.
13 LANXESS Product Information Sheet, Biochek® BIT 20, 2007-04-17.
14 Microbicides for the Protection of Materials, A Handbook Edited by Wilfried Paulus Part Two, p.659-661, 702-703.
15 LANXESS Product Information Sheet, Preventol® P100 Preservative, 2008-03-31.
16 Form No. 253-03194-09/19/13 PS Dow Microbial Product Brochure NeoloneTM 950 Preservative
18 Gillatt, J. The Effect of Redox Chemistry on the Efficacy of Biocides in Polymer Emulsions.
Contact Information:
Matthew Besterci, MATTHEW.BESTERCI@LANXESS.COM 412-809-1191
Cecilia M. McGough, CECILIA.MCGOUGH@LANXESS.COM 412-809-1042
As the industrial market shifts toward new and increasingly water-based polymer dispersions, the need for biocides to protect the integrity of the finished product increases. When fouled, polymer dispersions may exhibit a number of undesirable side-effects such as viscosity reduction, separation, and production of gas or odor. The diversity and complexity of these new systems presents a challenge to biocide selection. In industrial applications of polymer in water-based systems (e.g., coatings, adhesives, and sealants), analytical testing of the system enables a formulator to maximize biocide efficacy, thus protecting product integrity. Proper analytical screening including assay determination via High Performance Liquid Chromatography (HPLC), redox potential, pH measurement and general raw material compatibility testing helps in the development of an effective microbial protection program to inhibit or control the growth of microorganisms that cause biodeterioration. This paper addresses some key areas where analytical testing can provide critical information to ensure the optimal protection of polymer dispersions.
Introduction
Water-based polymer dispersions are colloidal polymer particles dispersed in water.1
The size of the colloidal polymer particles is usually in the range of 1 nm to 1 µm. The average industrial water-based polymer dispersions contain 40-60 percent colloid polymer particles. Typical industrial polymer dispersions are usually free-flowing with varying physical properties (e.g., rheology) based on the monomers used. These properties provide the functional backbone of the industrial application formulations. Polymer dispersions are major building blocks in the formulation of coatings, adhesives, paper coatings and other industrial products. Manufacturing and the composition of these polymer dispersions have been well documented in previous industry papers.1, 2, 3, 4
In general, water-based polymer dispersions have a high water content and high nutrient level providing ideal conditions for microbial growth. Microorganisms and their metabolic by-products can contribute to the breakdown of the polymer dispersion. Once the polymer dispersion has been contaminated, the properties and functionalities are affected. The use of biocides is critical to control and inhibit the growth of microorganisms within water-based polymer dispersions.
Important Test Methods
In the development of a biocide program for a polymer dispersion system, analytical testing can provide valuable information. Some tests are simple, cheap, and easy to perform while others are complex and require expensive equipment to obtain valuable data. Proactive analytical screening for pH and redox potential can be done very quickly and will aid in the selection of a proper biocide for a given system. HPLC is an effective way to monitor concentration of a biocide active ingredient. It is a more expensive and complicated form of testing, but when done properly, it is a powerful tool for verification of biocide compatibility and stability.
pH
The pH is defined as the negative logarithm of the hydrogen ion concentration. The numerical value is a ratio of the [H+] and [OH-] concentration in a given substance. In pure water at 25oC, the concentrations of [H+] and [OH-] are equal giving a pH value of seven, or neutral. Values below seven are acidic indicating a greater concentration of hydrogen ions, and values above seven are basic or alkaline indicating a greater concentration of hydroxyl ions.
When determining pH, some important factors must be considered regarding the selection of proper analytical equipment and test protocols. Choosing the right electrode is vitally important and there are many options. Basically, a pH measurement involves the use of two electrodes, the pH sensor and the reference electrode. Both electrodes are built into a single probe that is directly submerged into the sample solution. The most common reference system used is the silver/silver chloride system which consists of a silver wire coated with silver chloride in an electrolyte solution of potassium chloride saturated with silver chloride. Another factor in selecting the proper pH probe is the type of junction on the electrode. The junction is the opening in the reference electrode that maintains contact with the test sample. The sleeve junction is the best choice for testing dispersions due to the large contact area which reduces potential clogging of the junction by contaminant matter. Other specialized probes can offer performance advantages in extreme chemistries or temperatures.
Calibration of the pH meter is very important and usually involves either two or three buffer solutions preferably in or near the range of the test sample. Standard buffer solutions for polymer dispersions are at pH values of four, seven, and ten. Temperature has an effect on pH. Either a temperature probe should be used in conjunction with the pH probe or an attempt should be made to keep the temperature of the standard buffer solutions and test samples constant. Proper and frequent calibration will ensure that the readings are as accurate as possible.6
Redox Potential
Standard reduction potential (i.e., redox potential) is a measurement of the tendency of a substance to be reduced. Reduction is the gain of electrons. Thus, a measurement of redox potential is determining the likelihood that a sample will gain electrons; this is represented by a numerical value in millivolts (mV). The standard hydrogen electrode (SHE) is the basis for comparison of results. The redox potential of the SHE at room temperature is known to be zero. All results from various electrodes can be calibrated to read as the values that would be obtained with the SHE by using a conversion table from the manufacturer of the standard. Positive values indicate that the solution has oxidizing power, and negative values indicate reductive strength.8
As with pH probes, there are various electrodes that have attributes that are beneficial for specific test conditions. There are also various electrolyte solutions based on the ionic strength of the test matrix. It is important that the proper electrode is selected based on the polymer dispersion formulation. The recommended test protocols should be followed to ensure accuracy of the test results. Calibration using the ORP standard should be done before each test period to verify that the reported results are valid and that the device is working properly.
High Performance Liquid Chromatography
HPLC is an effective chromatographic process for the detection and quantification of biocide active ingredients. There are multiple parts of the process that add up to a powerful analytical technique. HPLC itself refers to the process and equipment that takes a sample and separates the components so that they can be collected or detected by various means. A sample is dissolved in solvent and forced through a tightly packed column at high pressure to enable separations with high resolution. Typically, ultraviolet-visible light detectors are coupled with HPLC systems through the use of a flow cell. The development of an effective HPLC method can be a long and complicated process, but it is the best method to monitor the compatibility of a biocide with a given system.
Sample preparation is the first and most critical step in the HPLC process. Polymer dispersions are a complex matrix from which to extract biocides. This step can be tackled with a mixture of research and trials. Solubility data in solvents for biocides is easily obtained and can start as a basis for the extraction process. The goal is to extract the biocide from the sample while removing contaminants. A good extraction solvent will contain all of the eluents in the chromatography process so that any reactions of the sample resulting in precipitates will occur prior to injection into the HPLC. The mixture of components in the solvent can be varied to achieve certain reactions. For example, an extraction of biocide from a polymer dispersion might cause the latex to crash out of the solution. The sample can then be centrifuged, and the liquid supernatant taken from the vial, thus removing the solid contaminants. Prior to injection, the sample should be passed through a sub-micron filter to ensure that no solid particles clog the column. A freeze-thaw process could also cause precipitate formation in a polymer dispersion sample. Solubility data and the eluent system are good information from which to base an extraction scheme but, ultimately, trial and error may lead to the best results overall.
Once a clean sample is acquired, the next step of the process comes into focus. The chromatography process is the most important part of the method development and may require the most time. Modern HPLC systems use reversed-phase (RP) chromatography which is an inversion of normal-phase chromatography. RP chromatography uses a non-polar packing material in the column and has a mobile phase that starts out polar and is shifted to a more non-polar mobile phase as is necessary. Based on an affinity for the mobile phase over the stationary phase, different components will be retained in the column for different amounts of time. Isocratic elution involves a mobile phase that does not change composition. Gradient elution involves multiple eluents (aqueous and organic, polar and non-polar) that are mixed in different ratios by a program in the machine (Figure 1). This allows for high resolution and shorter run times.10
Choosing the right column, flow rate, eluent compositions, and gradient profile can be a difficult process if done from scratch. Luckily, there are some standard starting points for biocides that can significantly shorten the development process. Nearly all of the biocides for polymer dispersions work well with reversed-phase systems composed of C18-packed columns and some mixture of acetonitrile and water as the non-polar and polar eluents respectively. As is seen in Figure 1, a typical gradient profile starts with a higher percentage of the aqueous phase and gradually shifts toward nearly an organic phase. The polar components in the sample will elute early due to a lack of affinity for the stationary phase, and the non-polar components will elute as the mobile phase shifts toward a mostly organic composition. Many hours can be spent optimizing this system to fit various needs; speed can be prioritized over resolution or the other way around. There might not be such a thing as a bad HPLC method as long as the results can be validated and are reproducible.
The final step of the HPLC process is the detection. If the wavelength of maximum absorption of the analyte is known, then this aspect of the test method is quite simple. If the wavelength of maximum detection is unknown for a certain analyte, and cannot be found in literature, then the process becomes more involved, and a more advanced detector might be required. Ultraviolet-Visible (UV-VIS) detection measures the absorption of wavelengths of light as they pass through a flow cell. As the components elute from the column, they pass through the flow cell absorbing light in the process. Absorbance is related to concentration. Therefore, quantification of the analyte can be achieved. The results are presented as a chromatogram, and determination of the area under the peak allows for concentration calculation (Figure 2).10
Only one wavelength can be measured using UV-VIS spectrometry so, if the proper wavelength is not known, this detection method is not ideal. A photodiode array detector (PDAD) operates similarly to a UV-VIS detector except that it can monitor a spectrum of wavelengths at once (Figure 3). Indeed, the PDAD can be used to determine the wavelength of maximum absorption of an analyte. Also, if there is interference from eluents or other components in the sample, the PDAD can be used to monitor the analyte at a wavelength where there is less interference.
Once all of the test method parameters have been adequately refined, it is important to validate that the method is accurate, precise, and reproducible. A typical analytical method validation for HPLC involves the use of standards with either multiple runs that can be averaged and/or a calibration curve with a linear fit line. One must first create a three-point calibration curve with a standard of known concentration and then test the calibration curve for accuracy, precision, reproducibility, and limit of detection. If the method/curve passes all of the tests, the method is validated, and there is assurance of the validity of the results.
Analytical Approach To Biocide Selection
Effective microbial protection of a polymer dispersion requires an in-depth look into the chemistries of both the matrix and the biocides. Knowing your chemistries, you can develop a scheme to simplify the selection process. Figure 4 outlines a systematic approach to biocide selection using the analytical tools referenced in the previous section.
Initial screening includes a review of the pH and redox potential of the polymer dispersion. These conditions provide the basic criteria from which to select the appropriate and most stable biocide under given conditions. The pH of a typical polymer dispersion ranges between 3.5 and 10.0. Ethylene vinyl acetate (EVA) and polyvinyl acetate (PVA) are examples of acidic polymer dispersions, whereas styrene butadiene is an example of a basic polymer dispersion. Most biocides are stable in slightly acidic to neutral pH ranges, but there are limited options in alkaline environments. For example, BIT shows efficacy across a broad range including pH 8.0 and above where there are limited options. Biocide options for polymer dispersions below pH 8.0 include bronopol, CMIT/MIT, and DBDCB. See Table 1 for more detailed information regarding effective pH ranges for biocides.
Redox reagents are critical for the dispersion polymerization process. However, residual levels of initiators and/or terminators (i.e., free radicals) in the cooled latex can impact the performance of the biocides due to the highly reactive nature of those chemistries. CMIT/MIT is susceptible to attack from reducing agents present in the cooled polymer matrix, thereby hindering the performance of the biocide. As opposed to CMIT/MIT, BIT can be unstable in oxidative environments. Monitoring the redox potential of the system during the manufacturing process can predict potential incompatibilities and determine the best time to incorporate the proper biocide system into the process. Table 1 provides common redox chemistries that impact biocide stability.
The next stage in the biocide selection process is the trial and error phase. The results from the initial screening (i.e., pH and redox data) provide the basis for the trials.
Compatibility trials are run with potential biocide candidates and evaluated by visual assessment. Precipitates, coagulation, and color changes are indicators of an incompatibility between the polymer dispersion system and the biocide. Yellowing could indicate a breakdown of the biocide. Coagulation is an indication that the electrostatic surfactant(s) may have been affected by the biocide. Polyvalent-salt stabilized biocides have been known to alter the properties of polymer dispersion systems. Monovalent stabilized biocides may be used as an alternative.
A lack of visual cues does not mean that the biocide is present and effective. Additional testing is needed to confirm microbial protection. Measurement of the active biocide concentration is the last step in the analytical approach to biocide selection. Biocides are only effective when used in the proper dosage levels for a given matrix.
One of the most powerful analytical tools for biocide detection is HPLC. Even though method development for HPLC can be a time-consuming process, the results obtained are vitally important to ensure microbial protection. The procedure for developing an HPLC method for biocide determination is outlined in the previous section. Using this procedure and additional information from the biocide manufacturer can demystify and simplify the development process significantly.
Conclusions
An analytical approach to biocide selection can save time and money for the polymer dispersion manufacturer. Redox potential and pH are simple and effective ways to perform an initial screening of the preservation program of a polymer dispersion system. They can provide basic guidelines for biocide selection, but further testing should be performed. A visual assessment of a dispersion after biocide addition may show coagulation or precipitation, thus indicating a possible incompatibility, or it may show no sign at all. HPLC can provide certainty about compatibility even if no outwardly visible signs of issues are present. It also confirms that the appropriate biocide dosage levels are present in the system.
Once the proper biocide system has been selected, microbial challenge testing should be performed to verify the biocide effectiveness. A basic principle to follow is that if the biocide is unstable in the polymer dispersion system, then microbial control is compromised. Therefore, it is crucial to confirm that the biocide is stable in the polymer dispersion system to allow for proper microbial control.
Disclaimer
The manner in which you use and the purpose to which you put and utilize our products, technical assistance and information (whether verbal, written or by way of production evaluations), including any suggested formulations and recommendations are beyond our control. Therefore, it is imperative that you test our products, technical assistance and information to determine to your own satisfaction whether they are suitable for your intended uses and applications. This application-specific analysis must at least include testing to determine suitability from a technical as well as health, safety, and environmental standpoint. Such testing has not necessarily been done by us. Unless we otherwise agree in writing, all products are sold strictly pursuant to the terms of our standard conditions of sale. All information and technical assistance is given without warranty or guarantee and is subject to change without notice. It is expressly understood and agreed that you assume and hereby expressly release us from all liability, in tort, contract or otherwise, incurred in connection with the use of our products, technical assistance, and information. Any statement or recommendation not contained herein is unauthorized and shall not bind us.
Nothing herein shall be construed as a recommendation to use any product in conflict with patents covering any material or its use. No license is implied or in fact granted under the claims of any patent.
1 Anderson C.D.; Daniels E.S. Emulsion Polymerisation and Latex Applications, ort 160, ISSN: 0889-3144 Volume Number 4, 2003.
2 McGough, C. M.; Nova-Ruiz, W.; Weigenand, O. The Fundamental of Choosing A Biocide to Protect a Polymer Dispersion including Technical, Environmental and Regulatory Issues.
3 Urban D.; Takamura, K. Polymer Dispersions and Their Industrial Application, 2002 Wiley VCH, p.6
4 Chern, C.S., Emulsion Polymerization Mechanisms and Kinectics, Progress in Polymer Science, Vol 31(2006) p. 443-486.
5 Hach Company. (2010). What Is pH And How Is It Measured? A Technical Handbook for Industry. Hach Company.p.6.
6 Mettler-Toledo AG. (2007, 10). A Guide to pH Measurement - The Theory and Practice of Laboratory pH Applications. Switzerland: Mettler-Toledo AG. p. 5-15.
7 Mettler-Toledo AG. (2007, 10). A Guide to pH Measurement - The Theory and Practice of Laboratory pH Applications. Switzerland: Mettler-Toledo AG. p. 18-26.
8 Harris, D. C. (2007). Quantitative Chemical Analysis (Seventh Edition ed.). New York, NY: W.H. Freeman and Company. p. 270-279.
9 Thermo Fisher Scientific Inc. (2007). User Guide: Redox/ORP Electrodes. p.6
10 Harris, D. C. (2007). Quantitative Chemical Analysis (Seventh Edition ed.). New York, NY: W.H. Freeman and Company. p. 556-583.
11 Erstling, K. Lanxess Deustchland, Leverkusen, Germany. Internal Communication, 2015.
12 Gillatt, J.W. The Microbial Spoilage of Polymer Dispersions and its Prevention in Directory of Microbicides for the Protection of Materials, A Handbook Edited by Wilfired Paulus p. 219.
13 LANXESS Product Information Sheet, Biochek® BIT 20, 2007-04-17.
14 Microbicides for the Protection of Materials, A Handbook Edited by Wilfried Paulus Part Two, p.659-661, 702-703.
15 LANXESS Product Information Sheet, Preventol® P100 Preservative, 2008-03-31.
16 Form No. 253-03194-09/19/13 PS Dow Microbial Product Brochure NeoloneTM 950 Preservative
18 Gillatt, J. The Effect of Redox Chemistry on the Efficacy of Biocides in Polymer Emulsions.
Contact Information:
Matthew Besterci, MATTHEW.BESTERCI@LANXESS.COM 412-809-1191
Cecilia M. McGough, CECILIA.MCGOUGH@LANXESS.COM 412-809-1042