George Brereton, Zhenya Zhu, Polina Ware, and Gerald King, Lanxess Corporation, Naugatuck, CT, USA, and Lanxess Corporation, Latina Italy01.20.20
Abstract
Superior blocked prepolymers have been developed based on the use of low free (LF) isocyanate urethane prepolymers and ε-Caprolactam (CAP). Low free isocyanate technology, which can achieve prepolymers with significantly lower viscosity, enables synthesis of prepolymers based on non-traditional raw materials such as para-phenylene diisocyanate (pPDI), more viscous polycarbonate polyols or unique amine types. This approach enables differentiated performance and properties unattainable with a conventional blocked prepolymer approach.
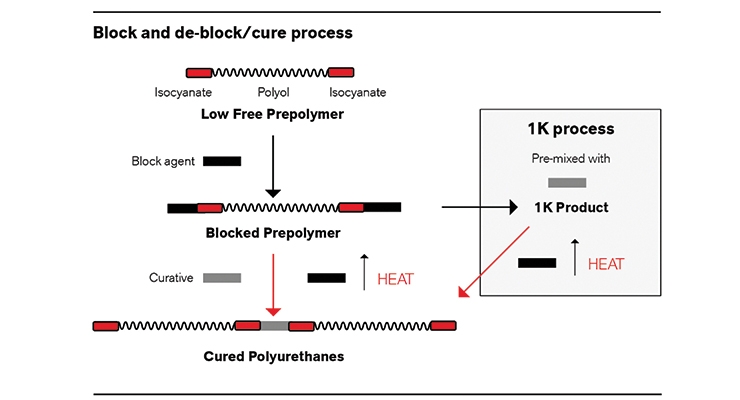
In blocked isocyanate prepolymers, all active isocyanate groups are temporarily converted into an unreactive state by reaction with a blocking agent. The blocking agent reacts with the active NCO groups making the prepolymer unreactive. Once heat is added to the blocked prepolymer, the reaction reverses and the active NCO groups can then start reacting with the chain extender. CAP can be used to block the NCO groups of a prepolymer, thereby creating an unreactive prepolymer at lower temperatures (23-70 °C). The CAP blocked prepolymer can then be mixed with fast reacting diamines and stored long-term until the mixture is heated to 100 oC-180 oC, allowing it to solidify and fully cure.
These blocked prepolymers can be supplied as 1K and 2K systems. A 1K system is created by adding a chain extender to the prepolymer. This stable 1K system, combined with LF technology provides processors with numerous advantages including simplified processing and product batch consistency by utilizing only one liquid stream with an established prepolymer/curative ratio. The benefits of 1K systems are advantageous in a number of industries including adhesives, coatings, binders and elastomers. For a 2K system approach, the blocked prepolymer can be used to increase the processors’ flexibility to use curatives that would otherwise be too reactive (i.e., aliphatic diamines). Using blocked prepolymer (or 1K) systems enables processors the control needed to work across many applications, whether the need is for a longer pot life or more control of the curing process.
The broad range of raw materials that can be used with blocked systems creates opportunities to make parts with exceptional properties that can perform at elevated temperatures and harsh conditions that otherwise would not be possible.
Introduction
The trend toward sustainability is driving innovation and new product development in the polyurethane industry. Sustainability is focused on developments that meet the current demands without compromising the ability of future generations to meet their needs, such as using greener and safer chemistries that meet increasingly strict regulatory requirements. Specific areas of innovation and growth in the PU industry include: recycling, solvent-free chemistries, green and bio-based materials, and non-isocyanate approaches, as well as blocked prepolymer technologies.1-8
In blocked prepolymer chemistry, all active isocyanates or active hydrogen-containing groups are made unreactive with the use of a blocking agent until the material is thermally unblocked. Typical blocking agents include phenols, nonylphenol (NP), methylethylketoxime (MEKO), alcohols, amides, imidazoles, pyrazoles and ε- Caprolactam (CAP). All blocked prepolymer systems can significantly decrease the amount of and, therefore, the risk of exposure to free isocyanates in elastomers, coatings, adhesives and sealants.4,9-10
In addition to 2K system where a curative is mixed with blocked prepolymer at a desired ratio and cured immediately, blocked prepolymers can also be prepackaged into a one component (1K) system with the addition of a fast reacting curative, thereby simplifying the processing of final elastomers (Figure 1). As ready to use formulations, 1K systems simplify the manufacturing process by eliminating the need for mixing equipment. These 1K systems provide batch-to-batch consistency, eliminate the need to mix prepolymer and curative components, and provide more processing flexibility, including longer pot life and more controlled curing.10-12
Our Urethane Systems business is extending its product range to superior CAP-blocked prepolymers and systems that are based on low free (LF) prepolymer technology, where the content of free isocyanate in the prepolymer can be reduced as low as below 0.1 percent. This technology is applied to prepolymers based on a wide variety of isocyanates, polyols and curatives including MDI (methylenediphenyl diisocyanate), TDI (toluene diisocyanate), HDI (hexamethylene diisocyanate) and pPDI (p-phenylene diisocyanate). CAP-blocked LF prepolymers can be synthesized with non-traditional raw materials, such as more viscous polycarbonate polyols or new amine types (such as MDEA, DETDA (diethylmethylbenzenediamine) or MCDEA (methylene bis(chloro-diethyl-aniline)), enabling enhanced performance and properties not possible with conventional blocked prepolymers.
Using LF isocyanate technology to produce prepolymers that are CAP blocked further improves the industrial hygiene and allows for systems with lower hazard classification.13-20
Experimental
Table 1 shows the raw materials that were used in this study. The blocked prepolymers were formed by reacting a prepolymer with a blocking agent until there was no active NCO. Prepolymers are the reaction product of diisocyanate and a polyol. Low free isocyanate prepolymers are prepolymers with residual diisocyanate removed. Generally, levels of free isocyanate can be less than 0.1% for TDI, MDI, HDI, and pPDI.
Viscosity data was measured using a Brookfield RVDV-11+ pro viscometer equipped with a thermocel heating system using a #27 spindle. Viscosity measurements were run on the prepolymer, the prepolymer after blocking, and the 1K system after the addition of the curative. These measurements allowed us to determine the stability of the 1K mixtures by measuring the viscosity after the mixture had aged in an oven for a specific time and temperature.
Physical samples were conditioned for at least 2 days at 72 °F and 50% humidity. These samples were then tested following ASTM procedures specifically ASTM D470 (split tear) and ASTM D412 (tensile testing). Shore A hardness was measured using ASTM D2240.
To test for heat and oil stability, the cured elastomers were aged for 3 weeks in ASTM #1 and #3 oil at 150 °C. After aging, the samples were conditioned and then tested following ASTM D470 and D412.
Dynamic properties were measured in torsion mode using TA Instruments ARES-G2 Rotational Rheometer with minimum transducer torque in oscillation = 0.05 μN.m and transducer torque resolution = 1 nN.m. It is equipped with a forced convection oven (FCO) which rapidly heats and cools the sample over the temperature range of -150 °C to 600 °C. Rectangular test specimens were used (approximately 50 mm x 11.8 mm x 3.2 mm). Dynamic temperature step experiments were performed by heating the sample from 30 °C to 180 °C with 10 °C step under an oscillatory strain of 4% and oscillation frequency of 10 Hz.
Samples were aged at least two weeks at ambient conditions before testing.
Results and Discussion
Ɛ -Caprolactam was chosen as a blocking agent for low free isocyanate polyols due to lower hazard classification, as depicted in Table 2. Ɛ-Caprolactam also has low volatility due to its higher boiling point, enabling the formation of thicker elastomer parts, and minimizing the formation of bubbles that can occur with more volatile blocking agents.
During the blocking process
prepolymer is blocked with Ɛ-Caprolactam, resulting in no risk of exposure to isocyanate from the product as supplied. This blocked system is stable up to 70 °C and has outstanding pour life of more than 18 hours at 60-80 °C processing temperatures based on curative choice. This system is fast curing; as an example a 4 mm thick part cures in five minutes at 180 °C.
Processing Ease 1K vs 2K
In addition to 2K systems where a curative is mixed with blocked prepolymer at a desired ratio, LF CAP prepolymers can also be prepackaged into a one component (1K) system with the addition of a fast reacting curative, thereby simplifying the processing of final elastomers. In elastomers, 1K blocked systems are primarily used for processing ease, in niche applications that require longer pot life or more controlled cure, as well as for performance, where a blocked system enables use of a fast acting curative that otherwise would not be possible. Herein, the material is already set at the desired prepolymer/curative ratio and all that is needed is to place the mixture in the necessary mold or location and add heat (Figure 2). Due to using fast reacting curatives, once the prepolymer or isocyanate deblocks it will react immediately. Then, the blocking agent will either evaporate out of the system or will stay in the matrix depending on the type of the agent used and the thickness of the final polyurethane component.
Benefit of LF to Lower Viscosity
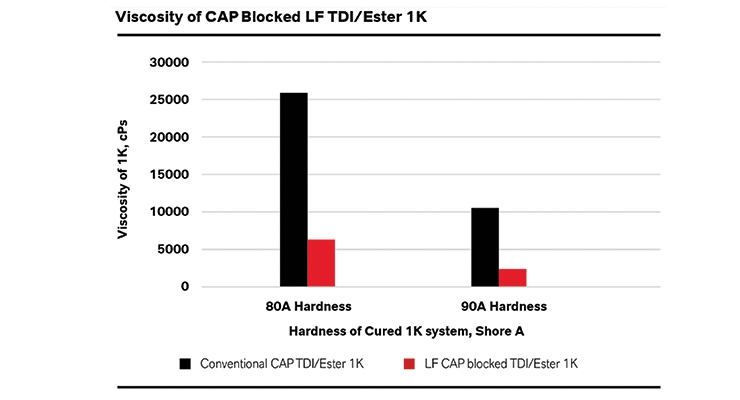
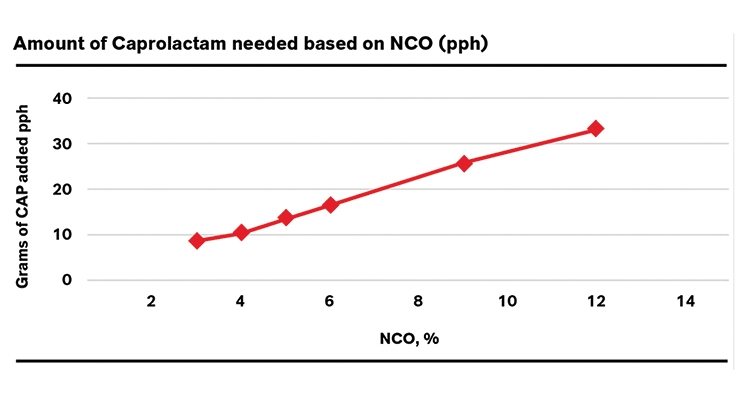
After a conventional prepolymer is blocked, its viscosity may increase significantly. However, combining blocked technology with a low free monomer approach creates a useable solution by lessening this effect as compared to a blocked conventional prepolymer. Since the excess monomers in the conventional prepolymers are capped with the blocking agent as well, they exhibit higher viscosity. The LF prepolymers lack the free monomer and the only NCO groups present end-cap prepolymer, thereby leading to increased processing ease. Figure 3 shows an example of the differences between LF and a conventional blocked prepolymer. As can be seen, LF-based systems have significantly lower viscosity than similar conventional systems. The conventional 80 shore A hardness system is viscous and is 25,800 cPS at 70 oC; in sharp contrast the LF monomer CAP-blocked prepolymer system is only 6150 cPs at the same temperature, thus the viscosity is reduced by the factor of 3. A similar trend is seen for 90 shore A hardness. Utilizing CAP-blocked LF prepolymers increases the ability to create harder elastomers created from blocked systems. In order to make harder elastomers, prepolymers of increasing NCO values are used. As shown in Figure 4, increasing the NCO also increases the amount of the blocking agent that is needed to fully block the system (more NCO groups). This makes it challenging to create high-hardness elastomers using blocked systems as they require large amounts of the blocking agent and tend to be extremely viscous.
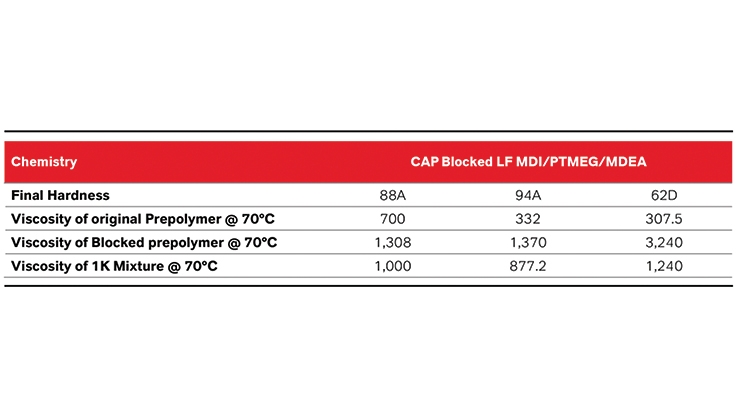
While viscosity at lower NCO’s is controlled more by the prepolymer itself (prepolymers are typically thicker at lower NCO values), the increase in viscosity that occurs when the prepolymer is blocked is highly dependent on the amount of the blocking agent that is used. Therefore, unlike typical prepolymers that exhibit viscosity decrease at higher NCO values, blocked system’s viscosities increase drastically. However, by implementing LF technology the viscosity increase can be managed via a less viscous starting prepolymer material. Table 3 shows three separate LF MDI/PTMEG mixed with MDEA systems that range from 88A and 62D and their respective viscosity. As can be seen the viscosity increases dramatically after the NCO groups are reacted with blocking agent. Due to low starting viscosity LF technology enables blocked systems that can be processed without use of plasticizers, solvents, or other processing aids that lower viscosity. Further, by making these systems into a 1K, the processablity of the system is increased. As the NCO increases the amount of curative needed increases as well, which leads to greater viscosity reduction in 1K systems. This is especially important in LF MDI systems that exhibit greater starting viscosity.
Performance
Using CAP-blocked LF prepolymer has a benefit of increasing the choice of raw materials that can be used in the system. Figure 5 depicts the breath of chemistries that can be used with LF CAP-blocked systems to achieve tailor-made high performance specialty urethanes.
For instance, CAP-blocked LF prepolymers using both pPDI and polycarbonate chemistries, can now be used. High performance of PPDI material is attributed to the compact, regular and symmetric structure of PPDI, which forms well-defined hard segment domains after reacting with chain extenders. LF prepolymers made from pPDI and polycarbonate provide excellent high temperature, solvent and oil resistance.13,21
Utilizing PPDI and polycarbonate raw materials, increases the toughness of the material. Figure 6 illustrates this with a comparison of LF PPDI/PC, alongside LFPPID/PCL, TDI/ester and HNBR which is a market standard for high temperature applications. The TDI ester was completely destroyed while HNBR lost a lot of strength. The LF PPDI/PC was by far the toughest while the LF PPDI/PCL had the higher temperature resistance but not the oil of the PC system.
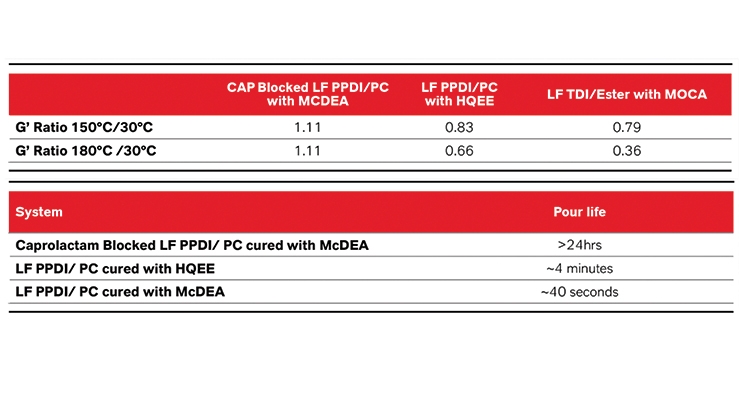
Desirable properties are formed when the LF PPDI/PC is cured with a diamine such as MCDEA. When curing an LF PPDI/PC with MCDEA, urea bonds are introduced which are higher melting and tougher, and create stronger crosslinks which allow the elastomers to outperform diol cured prepolymers. Such performance can be demonstrated by analyzing the three storage modulus of these materials (G’). Figure 7 shows a comparison of LF PPDI/PC cured with both HQEE and MCDEA together with an LF TDI/Ester cured with MOCA. In this chart it is apparent that MCDEA cure (black square) keeps its modulus all the way to 180 °C while the HQEE starts to drop off around 150 °C. The TDI/Ester + MOCA elastomer also drops off thus demonstrating the issue with using TDI in high temperature situations. The drop off in storage modulus is representative of the breakage of crosslinks and the on-set of deterioration of thermomechanical performance. The lower the ratio indicates a greater decline in storage modulus as the temperature increases.
However, as can be seen in Table 5, curing with MCDEA is not easy. This is where being able to block the material becomes important. Typically LFPDDI/PC/MCDEA has a reactivity of 30-40 seconds, which is extremely fast and can limit the manufacturers’ ability to work with the material. However, by blocking the material this is no longer a restricting factor. Manufacturers will be able to have over 24 hrs of working life at 70 °C, without worrying about curing the material prematurely. In addition to allowing the processors time to work with this system, it also allows them to make bigger parts, without worrying about filling the mold in time. The reaction will only take place once the necessary heat is added.
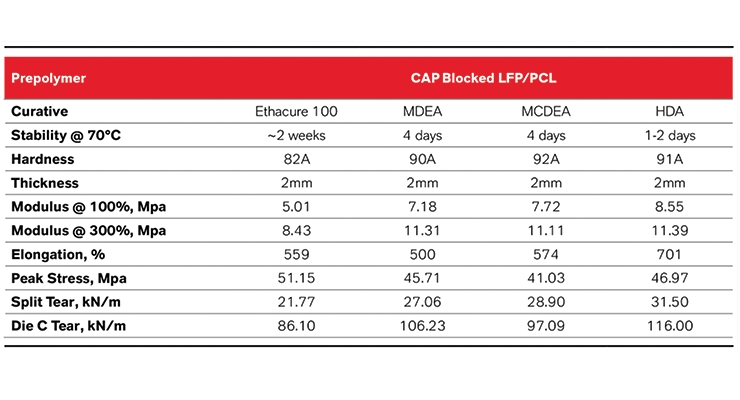
This benefit is seen not only with MCDEA, but with most fast acting diamines. Table 6 shows a blocked LF PPDI/PCL system that is mixed and then cured with 4 different curatives. The stability of the mixtures and the physical properties of the elastomers were then determined. All of these curatives could not be typically used with a LF PPDI/PCL prepolymer due to reactivity. However, due to the blocking even an aliphatic diamine such as HDA can be used. This opens up new possibilities that would otherwise be difficult to investigate.
Conclusion
LANXESS is at the forefront of blocked urethane systems technology with products for high performance elastomers. CAP-blocked LF products are superior one component (1K), blocked systems, developed based on low free isocyanate urethane prepolymers. They are designed to surpass conventional blocked systems to deliver enhanced performance, processing ease, and superior industrial hygiene.
These systems can be pre-mixed with all the components necessary to form an elastomer, including the chain extender, which is stable and ready to react when heated. CAP-blocked LF systems have lower hazard classifications for superior industrial hygiene, which is imparted by the choice of blocking agent and curative. They also provide batch- to-batch consistency, eliminate the need to mix prepolymer and curative components, and provide more processing flexibility, including longer pot life and more controlled curing. Low free isocyanate prepolymer technology enables these systems to be designed with significantly lower viscosity for easier processing and increased productivity. These systems enable the use of non-traditional raw materials, such as para-phenylene diisocyante (pPDI), more viscous polycarbonate polyols, and unique curative types, which would otherwise be too reactive to use in traditional systems. The ability to use such raw materials enables elastomers with exceptional properties that can perform at elevated temperatures and in harsh environments that would otherwise not
be possible.
CAP-blocked LF systems are superior systems that have enabled a new technological platform that delivers urethanes for the most demanding applications, improves productivity, and meets the highest health and safety standards.
References
1. Zhao, L., Recycling of polyurethane elastomer waste material. 2015, Shanxi Lingyun Polyurethane Co., Ltd., Peop. Rep. China. 2. Zhu, W., Method of recycling waste polyurethane products. 2015, Nanjing Meiding Technology Co., Ltd., Peop. Rep. China.
3. Nikje, M.M.A., Recycling of Polyurethane Wastes. 2016: Smithers Rapra Technology.
4. Rolph, M.S., et al., Blocked isocyanates: from analytical and experimental considerations to non- polyurethane applications. Polym. Chem., 2016. 7(48): p. 7351-7364.
5. Schmidt, S., et al., Isocyanate-Free Route to Poly(carbohydrate-urethane) Thermosets and 100% Bio-Based Coatings Derived from Glycerol Feedstock. Macromolecules (Washington, DC, U. S.), 2016. 49(19): p. 7268-7276.
6. Comi, M., et al., Adaptive bio-based polyurethane elastomers engineered by ionic hydrogen bonding interactions. Eur. Polym. J., 2017. 91: p. 408-419.
7. Cramail, H., E. Grau, and O. Lamarzelle. Isocyanate-free routes to polyurethanes and poly(hydroxyl urethane)s. 2017. American Chemical Society.
8. Farhadian, A., et al., Synthesis of fully bio-based and solvent free non-isocyanate poly (ester amide/urethane) networks with improved thermal stability on the basis of vegetable oils. Polym. Degrad. Stab., 2018. 155: p. 111-121.
9. Zhao, H.-j., T. Zhou, and Z.-x. Lin, Study on blocked isocyanates. Huaxue Yu Nianhe, 2015. 37(4): p. 291- 297.
10. Zhu, Z., G. King, and G. Brereton, Blocked isocyanate-terminated prepolymers with improved processing properties. 2018, USA.
11. Szycher, M., Szycher’s Handbook of Polyurethanes (2nd Edition). 2013, Taylor & Francis.
12. Clemitson, I.R., Castable Polyurethane Elastomers (2nd Edition). 2015, Taylor & Francis.
13. Zhu, Z., G. King, and G. Brereton, Cast urethanes made from low free monomer prepolymer with polycarbonate backbone and preparation of prepolymer. 2018, Lanxess Solutions Us Inc., USA. p. 26pp.
14. Rosenberg, R.O., et al. , Low Free TDI Prepolymers: Their Advantages, Limitations, and Fit. 1992.
15. Zhu, Z., et al. High performance polyurethane elastomers. 2005. American Chemical Society, Rubber Division.
16. Zhu, Z., et al. Novel thermoplastic polyurethane elastomers. 2013. American Chemical Society, Rubber Division.
17. Zhu, Z., et al., Novel thermoplastic polyurethane elastomers. Rubber World, 2013. 249(3): p. 20-27.
18. Zhu, Z. and R.O. Rosenberg, Thermoplastic polyurethane from low free monomer prepolymer. 2014, Chemtura Corporation, USA.
19. King, G., M. Timm, and P. Ware, Low-free prepolymers provide an excellent solution for planned EU restrictions on diisocyanates. FAPU, 2019(June 2019): p. 32-33.
20. Zhu, Z., et al., New Caprolactam-blocked prepolymers for 1K and 2K polyurethane reaction systems. PU Magazine, 2019(February/March 2019): p. 39-41.
21. Zhu, Z., J. Chin, and R.L. Palinkas. Advances in PPDI (para-phenylene diisocyanate) elastomers. 2000. Crain Communications Ltd. CW
Superior blocked prepolymers have been developed based on the use of low free (LF) isocyanate urethane prepolymers and ε-Caprolactam (CAP). Low free isocyanate technology, which can achieve prepolymers with significantly lower viscosity, enables synthesis of prepolymers based on non-traditional raw materials such as para-phenylene diisocyanate (pPDI), more viscous polycarbonate polyols or unique amine types. This approach enables differentiated performance and properties unattainable with a conventional blocked prepolymer approach.
In blocked isocyanate prepolymers, all active isocyanate groups are temporarily converted into an unreactive state by reaction with a blocking agent. The blocking agent reacts with the active NCO groups making the prepolymer unreactive. Once heat is added to the blocked prepolymer, the reaction reverses and the active NCO groups can then start reacting with the chain extender. CAP can be used to block the NCO groups of a prepolymer, thereby creating an unreactive prepolymer at lower temperatures (23-70 °C). The CAP blocked prepolymer can then be mixed with fast reacting diamines and stored long-term until the mixture is heated to 100 oC-180 oC, allowing it to solidify and fully cure.
These blocked prepolymers can be supplied as 1K and 2K systems. A 1K system is created by adding a chain extender to the prepolymer. This stable 1K system, combined with LF technology provides processors with numerous advantages including simplified processing and product batch consistency by utilizing only one liquid stream with an established prepolymer/curative ratio. The benefits of 1K systems are advantageous in a number of industries including adhesives, coatings, binders and elastomers. For a 2K system approach, the blocked prepolymer can be used to increase the processors’ flexibility to use curatives that would otherwise be too reactive (i.e., aliphatic diamines). Using blocked prepolymer (or 1K) systems enables processors the control needed to work across many applications, whether the need is for a longer pot life or more control of the curing process.
The broad range of raw materials that can be used with blocked systems creates opportunities to make parts with exceptional properties that can perform at elevated temperatures and harsh conditions that otherwise would not be possible.
Introduction
The trend toward sustainability is driving innovation and new product development in the polyurethane industry. Sustainability is focused on developments that meet the current demands without compromising the ability of future generations to meet their needs, such as using greener and safer chemistries that meet increasingly strict regulatory requirements. Specific areas of innovation and growth in the PU industry include: recycling, solvent-free chemistries, green and bio-based materials, and non-isocyanate approaches, as well as blocked prepolymer technologies.1-8
In blocked prepolymer chemistry, all active isocyanates or active hydrogen-containing groups are made unreactive with the use of a blocking agent until the material is thermally unblocked. Typical blocking agents include phenols, nonylphenol (NP), methylethylketoxime (MEKO), alcohols, amides, imidazoles, pyrazoles and ε- Caprolactam (CAP). All blocked prepolymer systems can significantly decrease the amount of and, therefore, the risk of exposure to free isocyanates in elastomers, coatings, adhesives and sealants.4,9-10
In addition to 2K system where a curative is mixed with blocked prepolymer at a desired ratio and cured immediately, blocked prepolymers can also be prepackaged into a one component (1K) system with the addition of a fast reacting curative, thereby simplifying the processing of final elastomers (Figure 1). As ready to use formulations, 1K systems simplify the manufacturing process by eliminating the need for mixing equipment. These 1K systems provide batch-to-batch consistency, eliminate the need to mix prepolymer and curative components, and provide more processing flexibility, including longer pot life and more controlled curing.10-12
Our Urethane Systems business is extending its product range to superior CAP-blocked prepolymers and systems that are based on low free (LF) prepolymer technology, where the content of free isocyanate in the prepolymer can be reduced as low as below 0.1 percent. This technology is applied to prepolymers based on a wide variety of isocyanates, polyols and curatives including MDI (methylenediphenyl diisocyanate), TDI (toluene diisocyanate), HDI (hexamethylene diisocyanate) and pPDI (p-phenylene diisocyanate). CAP-blocked LF prepolymers can be synthesized with non-traditional raw materials, such as more viscous polycarbonate polyols or new amine types (such as MDEA, DETDA (diethylmethylbenzenediamine) or MCDEA (methylene bis(chloro-diethyl-aniline)), enabling enhanced performance and properties not possible with conventional blocked prepolymers.
Using LF isocyanate technology to produce prepolymers that are CAP blocked further improves the industrial hygiene and allows for systems with lower hazard classification.13-20
Experimental
Table 1 shows the raw materials that were used in this study. The blocked prepolymers were formed by reacting a prepolymer with a blocking agent until there was no active NCO. Prepolymers are the reaction product of diisocyanate and a polyol. Low free isocyanate prepolymers are prepolymers with residual diisocyanate removed. Generally, levels of free isocyanate can be less than 0.1% for TDI, MDI, HDI, and pPDI.
Viscosity data was measured using a Brookfield RVDV-11+ pro viscometer equipped with a thermocel heating system using a #27 spindle. Viscosity measurements were run on the prepolymer, the prepolymer after blocking, and the 1K system after the addition of the curative. These measurements allowed us to determine the stability of the 1K mixtures by measuring the viscosity after the mixture had aged in an oven for a specific time and temperature.
Physical samples were conditioned for at least 2 days at 72 °F and 50% humidity. These samples were then tested following ASTM procedures specifically ASTM D470 (split tear) and ASTM D412 (tensile testing). Shore A hardness was measured using ASTM D2240.
To test for heat and oil stability, the cured elastomers were aged for 3 weeks in ASTM #1 and #3 oil at 150 °C. After aging, the samples were conditioned and then tested following ASTM D470 and D412.
Dynamic properties were measured in torsion mode using TA Instruments ARES-G2 Rotational Rheometer with minimum transducer torque in oscillation = 0.05 μN.m and transducer torque resolution = 1 nN.m. It is equipped with a forced convection oven (FCO) which rapidly heats and cools the sample over the temperature range of -150 °C to 600 °C. Rectangular test specimens were used (approximately 50 mm x 11.8 mm x 3.2 mm). Dynamic temperature step experiments were performed by heating the sample from 30 °C to 180 °C with 10 °C step under an oscillatory strain of 4% and oscillation frequency of 10 Hz.
Samples were aged at least two weeks at ambient conditions before testing.
Results and Discussion
Ɛ -Caprolactam was chosen as a blocking agent for low free isocyanate polyols due to lower hazard classification, as depicted in Table 2. Ɛ-Caprolactam also has low volatility due to its higher boiling point, enabling the formation of thicker elastomer parts, and minimizing the formation of bubbles that can occur with more volatile blocking agents.
During the blocking process
prepolymer is blocked with Ɛ-Caprolactam, resulting in no risk of exposure to isocyanate from the product as supplied. This blocked system is stable up to 70 °C and has outstanding pour life of more than 18 hours at 60-80 °C processing temperatures based on curative choice. This system is fast curing; as an example a 4 mm thick part cures in five minutes at 180 °C.
Processing Ease 1K vs 2K
In addition to 2K systems where a curative is mixed with blocked prepolymer at a desired ratio, LF CAP prepolymers can also be prepackaged into a one component (1K) system with the addition of a fast reacting curative, thereby simplifying the processing of final elastomers. In elastomers, 1K blocked systems are primarily used for processing ease, in niche applications that require longer pot life or more controlled cure, as well as for performance, where a blocked system enables use of a fast acting curative that otherwise would not be possible. Herein, the material is already set at the desired prepolymer/curative ratio and all that is needed is to place the mixture in the necessary mold or location and add heat (Figure 2). Due to using fast reacting curatives, once the prepolymer or isocyanate deblocks it will react immediately. Then, the blocking agent will either evaporate out of the system or will stay in the matrix depending on the type of the agent used and the thickness of the final polyurethane component.
Benefit of LF to Lower Viscosity
After a conventional prepolymer is blocked, its viscosity may increase significantly. However, combining blocked technology with a low free monomer approach creates a useable solution by lessening this effect as compared to a blocked conventional prepolymer. Since the excess monomers in the conventional prepolymers are capped with the blocking agent as well, they exhibit higher viscosity. The LF prepolymers lack the free monomer and the only NCO groups present end-cap prepolymer, thereby leading to increased processing ease. Figure 3 shows an example of the differences between LF and a conventional blocked prepolymer. As can be seen, LF-based systems have significantly lower viscosity than similar conventional systems. The conventional 80 shore A hardness system is viscous and is 25,800 cPS at 70 oC; in sharp contrast the LF monomer CAP-blocked prepolymer system is only 6150 cPs at the same temperature, thus the viscosity is reduced by the factor of 3. A similar trend is seen for 90 shore A hardness. Utilizing CAP-blocked LF prepolymers increases the ability to create harder elastomers created from blocked systems. In order to make harder elastomers, prepolymers of increasing NCO values are used. As shown in Figure 4, increasing the NCO also increases the amount of the blocking agent that is needed to fully block the system (more NCO groups). This makes it challenging to create high-hardness elastomers using blocked systems as they require large amounts of the blocking agent and tend to be extremely viscous.
While viscosity at lower NCO’s is controlled more by the prepolymer itself (prepolymers are typically thicker at lower NCO values), the increase in viscosity that occurs when the prepolymer is blocked is highly dependent on the amount of the blocking agent that is used. Therefore, unlike typical prepolymers that exhibit viscosity decrease at higher NCO values, blocked system’s viscosities increase drastically. However, by implementing LF technology the viscosity increase can be managed via a less viscous starting prepolymer material. Table 3 shows three separate LF MDI/PTMEG mixed with MDEA systems that range from 88A and 62D and their respective viscosity. As can be seen the viscosity increases dramatically after the NCO groups are reacted with blocking agent. Due to low starting viscosity LF technology enables blocked systems that can be processed without use of plasticizers, solvents, or other processing aids that lower viscosity. Further, by making these systems into a 1K, the processablity of the system is increased. As the NCO increases the amount of curative needed increases as well, which leads to greater viscosity reduction in 1K systems. This is especially important in LF MDI systems that exhibit greater starting viscosity.
Performance
Using CAP-blocked LF prepolymer has a benefit of increasing the choice of raw materials that can be used in the system. Figure 5 depicts the breath of chemistries that can be used with LF CAP-blocked systems to achieve tailor-made high performance specialty urethanes.
For instance, CAP-blocked LF prepolymers using both pPDI and polycarbonate chemistries, can now be used. High performance of PPDI material is attributed to the compact, regular and symmetric structure of PPDI, which forms well-defined hard segment domains after reacting with chain extenders. LF prepolymers made from pPDI and polycarbonate provide excellent high temperature, solvent and oil resistance.13,21
Utilizing PPDI and polycarbonate raw materials, increases the toughness of the material. Figure 6 illustrates this with a comparison of LF PPDI/PC, alongside LFPPID/PCL, TDI/ester and HNBR which is a market standard for high temperature applications. The TDI ester was completely destroyed while HNBR lost a lot of strength. The LF PPDI/PC was by far the toughest while the LF PPDI/PCL had the higher temperature resistance but not the oil of the PC system.
Desirable properties are formed when the LF PPDI/PC is cured with a diamine such as MCDEA. When curing an LF PPDI/PC with MCDEA, urea bonds are introduced which are higher melting and tougher, and create stronger crosslinks which allow the elastomers to outperform diol cured prepolymers. Such performance can be demonstrated by analyzing the three storage modulus of these materials (G’). Figure 7 shows a comparison of LF PPDI/PC cured with both HQEE and MCDEA together with an LF TDI/Ester cured with MOCA. In this chart it is apparent that MCDEA cure (black square) keeps its modulus all the way to 180 °C while the HQEE starts to drop off around 150 °C. The TDI/Ester + MOCA elastomer also drops off thus demonstrating the issue with using TDI in high temperature situations. The drop off in storage modulus is representative of the breakage of crosslinks and the on-set of deterioration of thermomechanical performance. The lower the ratio indicates a greater decline in storage modulus as the temperature increases.
However, as can be seen in Table 5, curing with MCDEA is not easy. This is where being able to block the material becomes important. Typically LFPDDI/PC/MCDEA has a reactivity of 30-40 seconds, which is extremely fast and can limit the manufacturers’ ability to work with the material. However, by blocking the material this is no longer a restricting factor. Manufacturers will be able to have over 24 hrs of working life at 70 °C, without worrying about curing the material prematurely. In addition to allowing the processors time to work with this system, it also allows them to make bigger parts, without worrying about filling the mold in time. The reaction will only take place once the necessary heat is added.
This benefit is seen not only with MCDEA, but with most fast acting diamines. Table 6 shows a blocked LF PPDI/PCL system that is mixed and then cured with 4 different curatives. The stability of the mixtures and the physical properties of the elastomers were then determined. All of these curatives could not be typically used with a LF PPDI/PCL prepolymer due to reactivity. However, due to the blocking even an aliphatic diamine such as HDA can be used. This opens up new possibilities that would otherwise be difficult to investigate.
Conclusion
LANXESS is at the forefront of blocked urethane systems technology with products for high performance elastomers. CAP-blocked LF products are superior one component (1K), blocked systems, developed based on low free isocyanate urethane prepolymers. They are designed to surpass conventional blocked systems to deliver enhanced performance, processing ease, and superior industrial hygiene.
These systems can be pre-mixed with all the components necessary to form an elastomer, including the chain extender, which is stable and ready to react when heated. CAP-blocked LF systems have lower hazard classifications for superior industrial hygiene, which is imparted by the choice of blocking agent and curative. They also provide batch- to-batch consistency, eliminate the need to mix prepolymer and curative components, and provide more processing flexibility, including longer pot life and more controlled curing. Low free isocyanate prepolymer technology enables these systems to be designed with significantly lower viscosity for easier processing and increased productivity. These systems enable the use of non-traditional raw materials, such as para-phenylene diisocyante (pPDI), more viscous polycarbonate polyols, and unique curative types, which would otherwise be too reactive to use in traditional systems. The ability to use such raw materials enables elastomers with exceptional properties that can perform at elevated temperatures and in harsh environments that would otherwise not
be possible.
CAP-blocked LF systems are superior systems that have enabled a new technological platform that delivers urethanes for the most demanding applications, improves productivity, and meets the highest health and safety standards.
References
1. Zhao, L., Recycling of polyurethane elastomer waste material. 2015, Shanxi Lingyun Polyurethane Co., Ltd., Peop. Rep. China. 2. Zhu, W., Method of recycling waste polyurethane products. 2015, Nanjing Meiding Technology Co., Ltd., Peop. Rep. China.
3. Nikje, M.M.A., Recycling of Polyurethane Wastes. 2016: Smithers Rapra Technology.
4. Rolph, M.S., et al., Blocked isocyanates: from analytical and experimental considerations to non- polyurethane applications. Polym. Chem., 2016. 7(48): p. 7351-7364.
5. Schmidt, S., et al., Isocyanate-Free Route to Poly(carbohydrate-urethane) Thermosets and 100% Bio-Based Coatings Derived from Glycerol Feedstock. Macromolecules (Washington, DC, U. S.), 2016. 49(19): p. 7268-7276.
6. Comi, M., et al., Adaptive bio-based polyurethane elastomers engineered by ionic hydrogen bonding interactions. Eur. Polym. J., 2017. 91: p. 408-419.
7. Cramail, H., E. Grau, and O. Lamarzelle. Isocyanate-free routes to polyurethanes and poly(hydroxyl urethane)s. 2017. American Chemical Society.
8. Farhadian, A., et al., Synthesis of fully bio-based and solvent free non-isocyanate poly (ester amide/urethane) networks with improved thermal stability on the basis of vegetable oils. Polym. Degrad. Stab., 2018. 155: p. 111-121.
9. Zhao, H.-j., T. Zhou, and Z.-x. Lin, Study on blocked isocyanates. Huaxue Yu Nianhe, 2015. 37(4): p. 291- 297.
10. Zhu, Z., G. King, and G. Brereton, Blocked isocyanate-terminated prepolymers with improved processing properties. 2018, USA.
11. Szycher, M., Szycher’s Handbook of Polyurethanes (2nd Edition). 2013, Taylor & Francis.
12. Clemitson, I.R., Castable Polyurethane Elastomers (2nd Edition). 2015, Taylor & Francis.
13. Zhu, Z., G. King, and G. Brereton, Cast urethanes made from low free monomer prepolymer with polycarbonate backbone and preparation of prepolymer. 2018, Lanxess Solutions Us Inc., USA. p. 26pp.
14. Rosenberg, R.O., et al. , Low Free TDI Prepolymers: Their Advantages, Limitations, and Fit. 1992.
15. Zhu, Z., et al. High performance polyurethane elastomers. 2005. American Chemical Society, Rubber Division.
16. Zhu, Z., et al. Novel thermoplastic polyurethane elastomers. 2013. American Chemical Society, Rubber Division.
17. Zhu, Z., et al., Novel thermoplastic polyurethane elastomers. Rubber World, 2013. 249(3): p. 20-27.
18. Zhu, Z. and R.O. Rosenberg, Thermoplastic polyurethane from low free monomer prepolymer. 2014, Chemtura Corporation, USA.
19. King, G., M. Timm, and P. Ware, Low-free prepolymers provide an excellent solution for planned EU restrictions on diisocyanates. FAPU, 2019(June 2019): p. 32-33.
20. Zhu, Z., et al., New Caprolactam-blocked prepolymers for 1K and 2K polyurethane reaction systems. PU Magazine, 2019(February/March 2019): p. 39-41.
21. Zhu, Z., J. Chin, and R.L. Palinkas. Advances in PPDI (para-phenylene diisocyanate) elastomers. 2000. Crain Communications Ltd. CW