Paul C. Fithian, Lindsay K. Holsen, Melissa A. Schwartz, Jessica Linker, BruggemannChemical U. S., Inc., Essential Polymers, L. Brüggemann GmbH & Co. KG04.15.20
Abstract
Reduction of residual free monomer is required in the manufacture of latexes to lower VOC emissions. This has become more critical as consumers are demanding coatings with reduced odor. Conventional post-polymerization chase techniques using reducing agent/oxidizer redox pairs can achieve low levels of residual monomers, but this can increase production time, add cost, and reduce production capacity.
A post-polymerization redox optimization study was conducted on a production latex made with a persulfate to thermally initiate the main polymerization. The monomers used were methyl methacrylate, styrene, methacrylic acid, and butyl acrylate. Various redox combinations were tested in the post-polymerization stage at different dosage levels and feed rates to minimize redox quantity, residual monomers, and time.
It was found that the right redox pair, fed simultaneously, was capable of reducing free monomer down to non-detectable levels in 15 minutes. This can enable up to 75% cycle time reduction for post-polymerization.
Introduction
The purpose of this study was to optimize redox pair, dosage, and feed rate in post-polymerization to remove residual monomers from a commercially produced waterborne latex. Essential Polymers of Merton, WI USA (Essential) supplied to L. Brüggemann GmbH & Co. KG, Heilbronn, Germany (Brüggemann) a latex containing the monomers methyl methacrylate (MMA), styrene (St), methacrylic acid (MAA), and butyl acrylate (BA). This latex was from a production sample that used a persulfate to thermally initiate and sustain the main polymerization.
After the initial lab results from Brüggemann were developed, Essential ran identical tests in their laboratories to confirm results.
To optimize residual monomer reduction of this emulsion polymer, various redox combinations were used in post-polymerization. Redox components were simultaneously metered into the latex over 30- or 60-minute periods to show the effect of the same total dosage over a shorter period. Latex samples were taken at timed intervals to record residual monomer reduction during post-polymerization. The results of each case were compared to determine the combination that provided the greatest reduction in residual monomer in the shortest time with the least amount of redox initiator.
Task
Redox post-polymerizations were performed on the supplied latex with various reducing agents. As received, the latex pH was 2.6 and remained unchanged during these optimization tests. In all cases, tert-butyl hydroperoxide (tBHP) was used as the oxidizer. Reducing agents used were Bruggolite® FF6 M (FF6), Bruggolite® E28 (E28), Bruggolite® TP 1646 (TP 1646), and sodium metabisulfite (SMBS). All post-polymerizations were performed at a constant temperature of 60 °C.
The test series was as follows:
1) Post-polymerization, uniform redox addition over a 60 minute period
2) Post-polymerization, uniform redox addition over a 30 minute period
3) Post-polymerization, one shot redox addition at time 0
Post-polymerization
Residual Monomer Content
Upon receipt, the residual monomer content of the styrene-acrylic latex was determined.
As expected, the presence of monomers MMA, St, MAA and BA were detected.
However, as BA was present at ~ 30,000 ppm, the concentrations of MMA, St, and MAA were not measured during the post-polymerization runs, as these were only contained in very small amounts. Since BA is harder to remove than the other monomers, emphasis was placed on BA measurement to quantify redox performance. Similar results have been shown with other hydrophobic monomers such as 2-ethylhexyl acrylate (2-EHA).
Reducing and Oxidizing Agents
Reducer
The reducing agent concentrations are expressed as weight percent of the total latex.
For example, all post-polymerizations were done with 350 g latex, and a total of 0.350 g reducing agent was used in the 0.1% tests.
Each reducing agent was used in the form of a 2% solution. The rate of solution addition was dependent on the total reducing agent used and the total time of addition; this rate was constant over the entire redox feed time.
Oxidizer
The oxidizer (tBHP) concentrations are expressed as weight percent of the total latex on a solids tBHP basis. For example, as all post-polymerizations were done with 350 g latex, 0.700 g tBHP was used in the 0.2% tests.
The tBHP was used in the form of a 2% solution on a tBHP solids basis, 7.14 g of a 70% tBHP solution (as supplied) was dissolved in 250 mL of deionized (DI) water. The rate of solution addition was dependent on the total tBHP used and the total time of addition; this rate was constant over the entire redox feed time.
Other Details
For each test, 350 g of latex was added to a jacketed vessel and tempered for 45 minutes to 60 °C, using a Julabo thermostat (Figure 1) with constant stirring at 285 rpm.
To initiate post-polymerization, simultaneous addition of the reducing agent and tBHP was started. The rate of redox dosing was controlled with separate Metrohm dosimeters, one for the reducing agent, and one for tBHP. The redox solutions were added uniformly over a total time duration, 30 or 60 minutes.
Note that in one of the runs, only tBHP was used to see if the oxidizer alone would remove residual monomers. As a reducing agent was not used, water was dosed simultaneously with the oxidizer to maintain the latex dilution at the same level.
In another run, 0.05% redox components were used as one-shot in relation to the mass of the latex (at 350 g latex 0.175 g). For this purpose, 8.75 mL of reducer or tBHP, with a maximum rate of 150 mL/min were dosed once the temperature reached 60 °C. Latex samples were taken at 5, 10, 15, and 30 minutes intervals to track the BA content.
Intermediate samples were taken with a disposable pipette. The first sample was taken from the latex before heating. After the latex reached 60 °C, another sample was taken, which marked the starting value after heating up. Subsequent samples were taken from the latex every 15 minutes during post-polymerization (~ 10 g each).
Monomer Determination
The residual monomer level was determined using Headspace-GC/MS. To accurately quantify the samples, a matrix-adjusted calibration (5-point calibration) of the GC/MS was performed before analyzing the latex samples. For this purpose, five different dilutions of the BA/St latex stock solution were prepared and analyzed with the help of the HS-GC/MS. The peak surfaces of the calibration standards were plotted against the monomer content in ppm (Figure 2).
To determine the residual monomer content, the post-polymerization samples were diluted with ultrapure water and analyzed using HS-GC/MS. During dilution, care was taken to ensure that the peak surfaces of the intermediate samples were within the peak surfaces of the calibration standards. Too large or too small a peak area in terms of calibration can lead to significant deviations in the measurement result.
Brüggemann Test Results
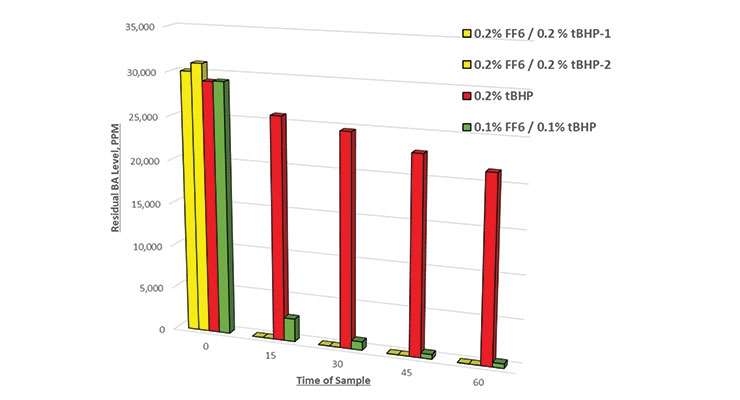
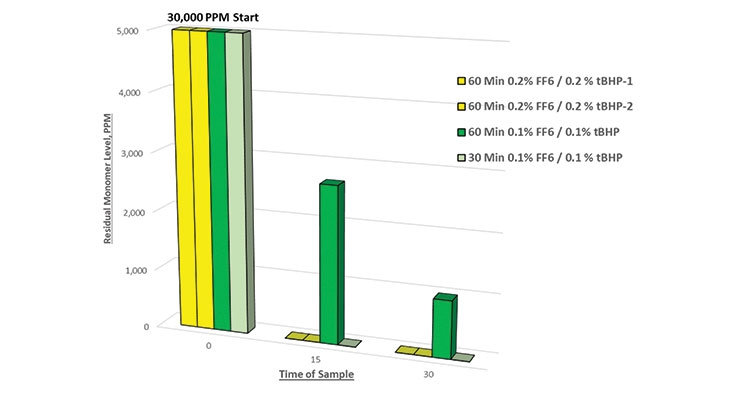
The results of all runs were tabulated to develop the following charts. Figure 3 shows a comparison of residual BA level with various dosages of FF6. In this series, one trial was done with tBHP only to highlight the fact that this oxidizer alone will not generate free radicals to remove residual monomers.
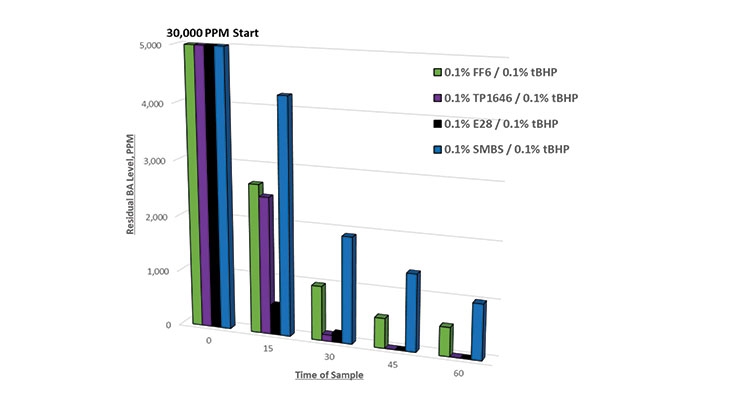
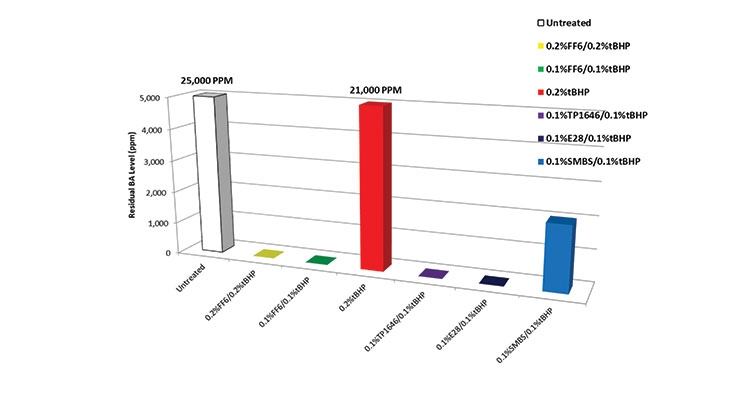
Figure 4 compares the residual BA levels with identical dosages of FF6, TP 1646, E28, and SMBS. Note the improved performance with E28 and TP 1646 compared to the other reducing agents. The dosages of each redox component were halved in this run to facilitate valid comparison of the reducing agents. Note again the superior performance of TP 1646 and E28 over the other reducing agents. This improved performance can be directly corelated to the improved stability of these two products in acidic conditions.
Post-polymerization Time 30 Minutes
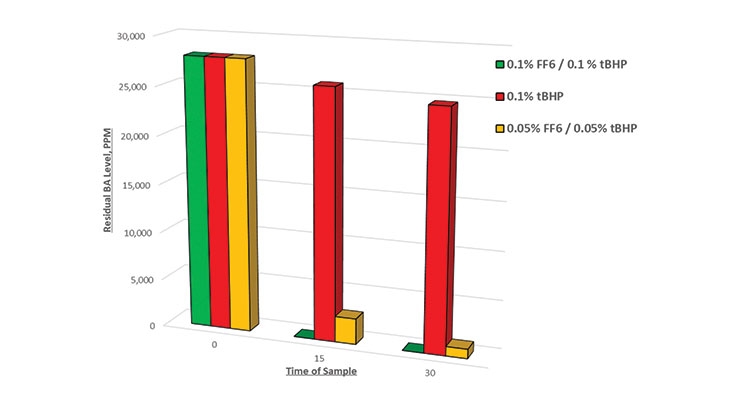
The results from Figure 3 reveal that the residual monomer level dropped to zero after 15 minutes at a dosage of 0.2% FF6. A new trial was run to dose 0.1% FF6 over a 30-minute time period, which is equivalent to a dosage of 0.2% FF6 over a 60-minute time period. The results shown in Figure 5 reveal that a lower dosage over a shorter time period will result in complete removal of residual monomers. Note again that there was virtually no reduction in residual BA level when tBHP was employed without a reducing agent.
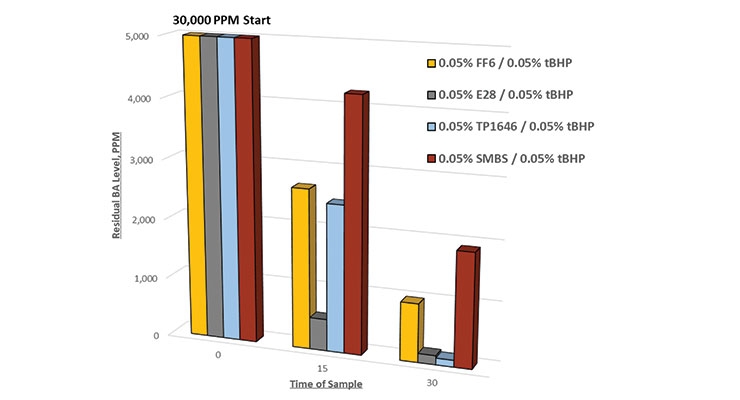
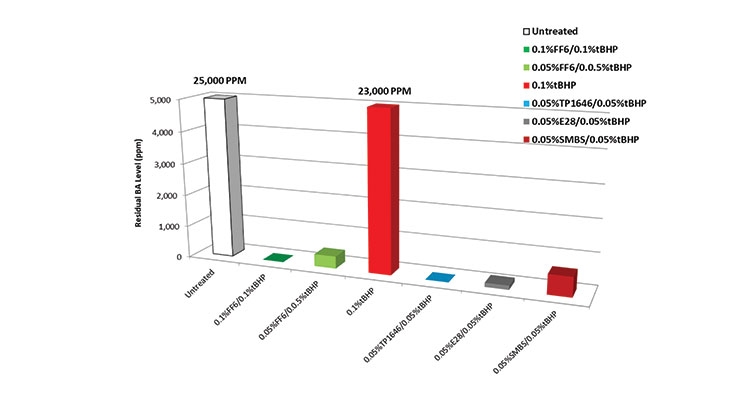
The next trial was run to compare different reducing agents applied in a chase time of 30 minutes, this time at an even lower dosage of 0.05%. Figure 6 shows again the superior reduction in residual BA level with E28 and TP 1646.
Post-Polymerization Time - 60 versus 30 Minutes
Figure 7 extracts data from the previous charts to compare FF6/tBHP dosage and time after 30 minutes of redox addition. It reveals that dosing a total of 0.1% over a 60-minute period is not nearly as effective as dosing the same total amount over a 30-minute period. This is a key point for redox optimization in emulsion polymer chase: Faster addition of redox results in a higher concentration of free radicals in the latex, substantially improving free monomer conversion. It enables lower free monomer in less time at lower dosage.
In three of these examples, all BA monomer is removed after 15 minutes, with a total dosage of 0.05%. Optimization of redox can enable process time reduction of 45 minutes or more in a typical emulsion polymer chase.
Post-polymerization - Effect of One-Shot Addition of Redox
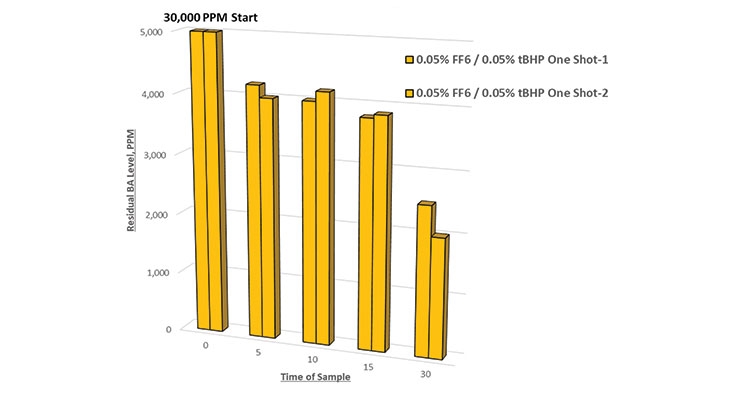
If a shorter time period of uniform redox addition will enable lower total dosage of the redox components, a theory was proposed to test effectiveness of adding redox in one shot at the start of post-polymerization. Figure 8 shows the results of this; it reveals much less residual BA reduction. Our theory on this is that radicals generated via redox combination are most effective at attacking residual monomers when a preponderance of items around a free radical are residual monomers. This is only possible with uniform redox addition in a well agitated system. When all redox components are “dumped” in, it is more likely that the generated radical will be consumed by an adjacent radical and destroyed, preventing it from attacking a residual monomer.
Essential Test Results
Residual Monomer Measurements
Upon review of the results generated by Brüggemann, Essential initiated tests using the same methods and dosage to confirm the results of residual BA monomer at the end of the chase session. Figure 9 shows that adding redox components over a 60-minute chase period showed even better performance of the 0.1% FF6/tBHP combination than found by Brüggemann. It also confirms virtually no residual BA reduction when tBHP is used alone.
Figure 10 shows the residual BA level when redox components were added over a 30-minute chase period. The results differed slightly, but generally tracked those from the Brüggemann tests.
Finished Product Performance Results
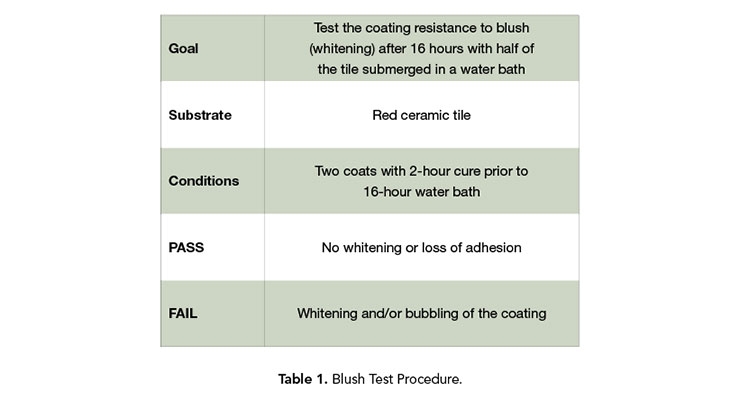
The resulting residual BA level with these techniques and reducing agents are excellent, but it is necessary to check if the finished polymer will exhibit any negative performance attributes. A blush test was chosen for this, following the procedure listed in Table 1.
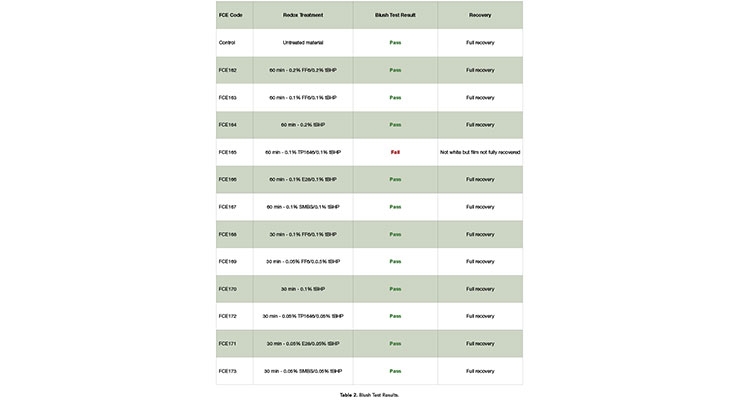
The results of this blush test on samples created from the redox dosage study are presented in Table 2. All but one of the combinations passed the test. The reason for this failure has not been determined, and it may be an anomaly. The test was only performed once; it is possible that a second test of the 0.1% TP 1646/0.1% tBHP may pass the test.
Conclusion
With redox-initiated post-polymerization, the level of residual BA can be reduced to undetectable levels in as little as 15 minutes. The amount of redox components and time period of redox addition were shown to be related. Simultaneous redox addition over a shorter time period will minimize residual monomers, redox dosage, and cycle time.
One shot addition of all redox components at the start of post-polymerization has been shown to be less effective at reducing residual monomers. Use of tBHP without a reducing agent also had little or no potential to reduce residual monomers.
All the Bruggolite® reducing agents showed excellent capability to quickly reduce or eliminate residual BA in redox chase.
For more information: paul.fithian@brueggemann.com, (219) 448-9114
Reduction of residual free monomer is required in the manufacture of latexes to lower VOC emissions. This has become more critical as consumers are demanding coatings with reduced odor. Conventional post-polymerization chase techniques using reducing agent/oxidizer redox pairs can achieve low levels of residual monomers, but this can increase production time, add cost, and reduce production capacity.
A post-polymerization redox optimization study was conducted on a production latex made with a persulfate to thermally initiate the main polymerization. The monomers used were methyl methacrylate, styrene, methacrylic acid, and butyl acrylate. Various redox combinations were tested in the post-polymerization stage at different dosage levels and feed rates to minimize redox quantity, residual monomers, and time.
It was found that the right redox pair, fed simultaneously, was capable of reducing free monomer down to non-detectable levels in 15 minutes. This can enable up to 75% cycle time reduction for post-polymerization.
Introduction
The purpose of this study was to optimize redox pair, dosage, and feed rate in post-polymerization to remove residual monomers from a commercially produced waterborne latex. Essential Polymers of Merton, WI USA (Essential) supplied to L. Brüggemann GmbH & Co. KG, Heilbronn, Germany (Brüggemann) a latex containing the monomers methyl methacrylate (MMA), styrene (St), methacrylic acid (MAA), and butyl acrylate (BA). This latex was from a production sample that used a persulfate to thermally initiate and sustain the main polymerization.
After the initial lab results from Brüggemann were developed, Essential ran identical tests in their laboratories to confirm results.
To optimize residual monomer reduction of this emulsion polymer, various redox combinations were used in post-polymerization. Redox components were simultaneously metered into the latex over 30- or 60-minute periods to show the effect of the same total dosage over a shorter period. Latex samples were taken at timed intervals to record residual monomer reduction during post-polymerization. The results of each case were compared to determine the combination that provided the greatest reduction in residual monomer in the shortest time with the least amount of redox initiator.
Task
Redox post-polymerizations were performed on the supplied latex with various reducing agents. As received, the latex pH was 2.6 and remained unchanged during these optimization tests. In all cases, tert-butyl hydroperoxide (tBHP) was used as the oxidizer. Reducing agents used were Bruggolite® FF6 M (FF6), Bruggolite® E28 (E28), Bruggolite® TP 1646 (TP 1646), and sodium metabisulfite (SMBS). All post-polymerizations were performed at a constant temperature of 60 °C.
The test series was as follows:
1) Post-polymerization, uniform redox addition over a 60 minute period
2) Post-polymerization, uniform redox addition over a 30 minute period
3) Post-polymerization, one shot redox addition at time 0
Post-polymerization
Residual Monomer Content
Upon receipt, the residual monomer content of the styrene-acrylic latex was determined.
As expected, the presence of monomers MMA, St, MAA and BA were detected.
However, as BA was present at ~ 30,000 ppm, the concentrations of MMA, St, and MAA were not measured during the post-polymerization runs, as these were only contained in very small amounts. Since BA is harder to remove than the other monomers, emphasis was placed on BA measurement to quantify redox performance. Similar results have been shown with other hydrophobic monomers such as 2-ethylhexyl acrylate (2-EHA).
Reducing and Oxidizing Agents
Reducer
The reducing agent concentrations are expressed as weight percent of the total latex.
For example, all post-polymerizations were done with 350 g latex, and a total of 0.350 g reducing agent was used in the 0.1% tests.
Each reducing agent was used in the form of a 2% solution. The rate of solution addition was dependent on the total reducing agent used and the total time of addition; this rate was constant over the entire redox feed time.
Oxidizer
The oxidizer (tBHP) concentrations are expressed as weight percent of the total latex on a solids tBHP basis. For example, as all post-polymerizations were done with 350 g latex, 0.700 g tBHP was used in the 0.2% tests.
The tBHP was used in the form of a 2% solution on a tBHP solids basis, 7.14 g of a 70% tBHP solution (as supplied) was dissolved in 250 mL of deionized (DI) water. The rate of solution addition was dependent on the total tBHP used and the total time of addition; this rate was constant over the entire redox feed time.
Other Details
For each test, 350 g of latex was added to a jacketed vessel and tempered for 45 minutes to 60 °C, using a Julabo thermostat (Figure 1) with constant stirring at 285 rpm.
To initiate post-polymerization, simultaneous addition of the reducing agent and tBHP was started. The rate of redox dosing was controlled with separate Metrohm dosimeters, one for the reducing agent, and one for tBHP. The redox solutions were added uniformly over a total time duration, 30 or 60 minutes.
Note that in one of the runs, only tBHP was used to see if the oxidizer alone would remove residual monomers. As a reducing agent was not used, water was dosed simultaneously with the oxidizer to maintain the latex dilution at the same level.
In another run, 0.05% redox components were used as one-shot in relation to the mass of the latex (at 350 g latex 0.175 g). For this purpose, 8.75 mL of reducer or tBHP, with a maximum rate of 150 mL/min were dosed once the temperature reached 60 °C. Latex samples were taken at 5, 10, 15, and 30 minutes intervals to track the BA content.
Intermediate samples were taken with a disposable pipette. The first sample was taken from the latex before heating. After the latex reached 60 °C, another sample was taken, which marked the starting value after heating up. Subsequent samples were taken from the latex every 15 minutes during post-polymerization (~ 10 g each).
Monomer Determination
The residual monomer level was determined using Headspace-GC/MS. To accurately quantify the samples, a matrix-adjusted calibration (5-point calibration) of the GC/MS was performed before analyzing the latex samples. For this purpose, five different dilutions of the BA/St latex stock solution were prepared and analyzed with the help of the HS-GC/MS. The peak surfaces of the calibration standards were plotted against the monomer content in ppm (Figure 2).
To determine the residual monomer content, the post-polymerization samples were diluted with ultrapure water and analyzed using HS-GC/MS. During dilution, care was taken to ensure that the peak surfaces of the intermediate samples were within the peak surfaces of the calibration standards. Too large or too small a peak area in terms of calibration can lead to significant deviations in the measurement result.
Brüggemann Test Results
The results of all runs were tabulated to develop the following charts. Figure 3 shows a comparison of residual BA level with various dosages of FF6. In this series, one trial was done with tBHP only to highlight the fact that this oxidizer alone will not generate free radicals to remove residual monomers.
Figure 4 compares the residual BA levels with identical dosages of FF6, TP 1646, E28, and SMBS. Note the improved performance with E28 and TP 1646 compared to the other reducing agents. The dosages of each redox component were halved in this run to facilitate valid comparison of the reducing agents. Note again the superior performance of TP 1646 and E28 over the other reducing agents. This improved performance can be directly corelated to the improved stability of these two products in acidic conditions.
Post-polymerization Time 30 Minutes
The results from Figure 3 reveal that the residual monomer level dropped to zero after 15 minutes at a dosage of 0.2% FF6. A new trial was run to dose 0.1% FF6 over a 30-minute time period, which is equivalent to a dosage of 0.2% FF6 over a 60-minute time period. The results shown in Figure 5 reveal that a lower dosage over a shorter time period will result in complete removal of residual monomers. Note again that there was virtually no reduction in residual BA level when tBHP was employed without a reducing agent.
The next trial was run to compare different reducing agents applied in a chase time of 30 minutes, this time at an even lower dosage of 0.05%. Figure 6 shows again the superior reduction in residual BA level with E28 and TP 1646.
Post-Polymerization Time - 60 versus 30 Minutes
Figure 7 extracts data from the previous charts to compare FF6/tBHP dosage and time after 30 minutes of redox addition. It reveals that dosing a total of 0.1% over a 60-minute period is not nearly as effective as dosing the same total amount over a 30-minute period. This is a key point for redox optimization in emulsion polymer chase: Faster addition of redox results in a higher concentration of free radicals in the latex, substantially improving free monomer conversion. It enables lower free monomer in less time at lower dosage.
In three of these examples, all BA monomer is removed after 15 minutes, with a total dosage of 0.05%. Optimization of redox can enable process time reduction of 45 minutes or more in a typical emulsion polymer chase.
Post-polymerization - Effect of One-Shot Addition of Redox
If a shorter time period of uniform redox addition will enable lower total dosage of the redox components, a theory was proposed to test effectiveness of adding redox in one shot at the start of post-polymerization. Figure 8 shows the results of this; it reveals much less residual BA reduction. Our theory on this is that radicals generated via redox combination are most effective at attacking residual monomers when a preponderance of items around a free radical are residual monomers. This is only possible with uniform redox addition in a well agitated system. When all redox components are “dumped” in, it is more likely that the generated radical will be consumed by an adjacent radical and destroyed, preventing it from attacking a residual monomer.
Essential Test Results
Residual Monomer Measurements
Upon review of the results generated by Brüggemann, Essential initiated tests using the same methods and dosage to confirm the results of residual BA monomer at the end of the chase session. Figure 9 shows that adding redox components over a 60-minute chase period showed even better performance of the 0.1% FF6/tBHP combination than found by Brüggemann. It also confirms virtually no residual BA reduction when tBHP is used alone.
Figure 10 shows the residual BA level when redox components were added over a 30-minute chase period. The results differed slightly, but generally tracked those from the Brüggemann tests.
Finished Product Performance Results
The resulting residual BA level with these techniques and reducing agents are excellent, but it is necessary to check if the finished polymer will exhibit any negative performance attributes. A blush test was chosen for this, following the procedure listed in Table 1.
The results of this blush test on samples created from the redox dosage study are presented in Table 2. All but one of the combinations passed the test. The reason for this failure has not been determined, and it may be an anomaly. The test was only performed once; it is possible that a second test of the 0.1% TP 1646/0.1% tBHP may pass the test.
Conclusion
With redox-initiated post-polymerization, the level of residual BA can be reduced to undetectable levels in as little as 15 minutes. The amount of redox components and time period of redox addition were shown to be related. Simultaneous redox addition over a shorter time period will minimize residual monomers, redox dosage, and cycle time.
One shot addition of all redox components at the start of post-polymerization has been shown to be less effective at reducing residual monomers. Use of tBHP without a reducing agent also had little or no potential to reduce residual monomers.
All the Bruggolite® reducing agents showed excellent capability to quickly reduce or eliminate residual BA in redox chase.
For more information: paul.fithian@brueggemann.com, (219) 448-9114