Shiying Zheng and Sudhir Ananthachar, Evonik Corporation04.18.22
Abstract
Environmental regulations and demands for worker and end-user safety have driven the coatings industry to develop products in compliance with low VOC requirements. High performance waterborne urethane-acrylic hybrid polymer dispersions (HPDs) have been developed to offer cost and performance advantages over standard 1K coating materials such as polyurethane dispersions (PUDs), acrylic emulsions, or blends of the two. Although, both PUDs and HPDs provide many benefits including superior mechanical properties and chemical resistance, one disadvantage is the inclusion of N-methylpyrrolidone (NMP) solvent, which is commonly used as a necessary process solvent. This paper presents the development of NMP-free HPDs with outstanding performance similar to the analogous NMP-containing HPDs. These HPDs are true hybrids characterized by an interpenetrating network (IPN) structure as a result of urethane and acrylic polymerized as a homogenous mixture. The IPN structure contributes to the unique performance attribute of the HPDs compared to acrylic/PUD blends.The NMP-free nature of these HPDs with the proper surfactant combination offers flexibility in formulating high performance coatings with low VOC. The formulation, application, and performance properties of these HPDs will be discussed in this paper.
Introduction
Coating industry is constantly innovating to provide new technologies and solutions to meet the more stringent low emission requirements and key market trends for productivity improvement and cost reduction while maintaining high coating performance.
Technology advancements has equipped the industry with new waterborne systems that deliver fast dry speed, simplified coating application, and better performance. Waterborne technology has gained wide acceptance as an environmentally friendly alternative to solvent borne system and has become commercially important due to low volatile organic content (VOC) and excellent coating properties.
Two of the most popular waterborne coating systems are acrylic and polyurethane dispersions.1,2 Acrylic dispersions provide coatings with good weathering properties, well understood structure-property relationships, and relatively low cost.
Polyurethanes are well known for their excellent balance of mechanical toughness and chemical resistance. Their superior physical and chemical properties have been attributed to a combination of their molecular structure and hard/soft domain morphology.3-5 Coatings from the waterborne polyurethane dispersions (PUDs) preserve this unique morphology typically achieved through solvent borne systems and maintain polyurethane’s excellent coating performance without using solvent.
Waterborne polyurethane dispersions are popular choices for a variety of one-component coatings for wood such as flooring and furniture, plastic, leather, metal, and concrete due to their outstanding coating properties.
In addition to the excellent mechanical and chemical properties of typical polyurethanes, aliphatic PUDs offer superior UV resistance where exposure to direct or indirect sunlight occurs. However, one of the main disadvantages of the aliphatic PUDs is their relatively high cost. This has motivated formulators to find ways to reduce the cost of their coatings by blending the high cost PUD with the low cost acrylic polymer dispersions, which is about half the price of a standard aliphatic PUD. Although the acrylics reduce the system cost, they also reduce the overall performance of the coating. The reduction in performance can be lower than what would be predicted from a linear arithmetic rule of mixtures.6,7
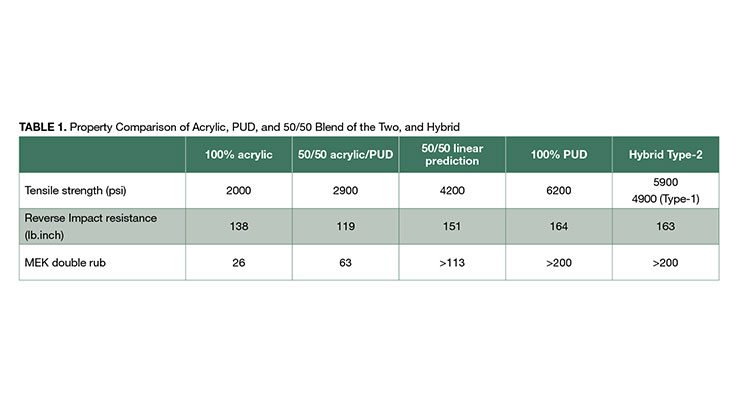
Data in Table 1 compares the properties of acrylic, PUD, and a 50/50 acrylic/PUD blend. The polyurethane has significantly higher tensile strength than the acrylic polymer. Interestingly, the tensile strength of the 50/50 acrylic/PUD blend is only ~2900 psi, considerably lower than the ~4200 psi predicted by a linear rule of mixture. Similar undesirable effects were observed with other mechanical and chemical resistance properties. One possible reason for this behavior is that, on a molecular level, the acrylic polymers and polyurethanes are not miscible with each other. As a result, the polymers remain phase-separated during film formation and this phase morphology is probably responsible for the diminished performance behavior shown in Table 1.
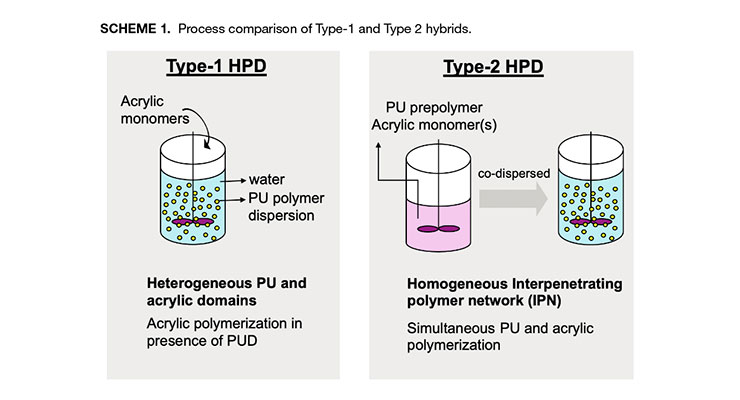
To overcome the phase separation issue of the acrylic/PUD blend, hybrid polymer dispersions (HPD) have been developed to take advantage of the low cost of the acrylics and the outstanding mechanical properties of PUD. The hybrids incorporate both the urethane and the acrylic polymers into the same dispersion. There are two main approaches to produce hybrid materials. The most representative approach is to polymerize acrylic monomers in the presence of a polyurethane dispersion to obtain a hybrid dispersion designated as Type-1 hybrid.8 The other approach is the in-situ method where the polyurethane prepolymer and acrylic monomers are dispersed in water, and then polymerize concurrently in water to form a hybrid material with intimately entangled acrylic and polyurethane chains, like an interpenetrating network (IPN) structure.7, 9, 10 This is designated as Type-2 hybrid. The comparison of the two approaches is illustrated in Scheme 1.
Both hybrid processes result in improved molecular compatibility compared to the simple blend. Some level of intimately mixing at the molecular level of acrylic and polyurethane chains and the polymers are presumably held together via chain entanglements and secondary intermolecular bonding forces. A minor extent of grafting between urethane and acrylic chains is also possible. One benefit of the hybrid processes is the increased tensile strength of the resulting materials as compared to its blended counterpart as shown in Table 1. The hybrid systems showed higher tensile strength than the predicted value, and well above that of the blend. In fact, Type 2 hybrid has tensile strength of approximately 5900 psi, higher than that of Type 1 of 4900, and nearly as high as that of the 100% polyurethane. The effect of improved performance of hybrids over physical blends is also apparent with other properties such as toughness, durability, and chemical/solvent resistance as shown in Table 1.
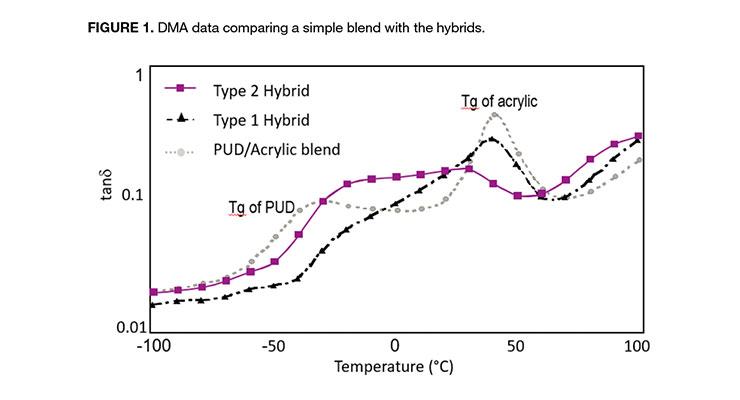
The improved compatibility is demonstrated by the dynamic mechanical analysis (DMA) data shown in Figure 1.
The simple blend has two distinct tan delta (tanδ) peaks, corresponding to the glass transition temperatures (Tg) for the phase-separated urethane and acrylic polymers. Type-1 hybrid also shows two Tg peaks, but the peaks have become broader, indicative of some limited level of molecular mixing. In contrast, a Type-2 hybrid exhibits only a single, very broad tanδ peak. The single peak, which spans the temperature range between the theoretical Tgs of the urethane and acrylic polymers, is consistent with an interpenetrating network (IPN) structure. From the data presented in Table 1, it is evident that the phase morphology of a urethane/acrylic polymer system has a significant influence on the ultimate performance.
HPDs and PUDs are typically prepared in a polar aprotic solvent such as N-methylpyrrolidone (NMP), which is required in the polyurethane prepolymer synthesis step to improve solubility of the acid diol monomer and reduce viscosity of prepolymer. Due to its relatively high boiling point, NMP remains in the final dispersion and the levels range from 10% to 15% for PUDs and 3% to 8% for HPDs. NMP is beneficial as a coalescing solvent for film formation in coatings. However, NMP is classified in category 1B reproductive toxicity and coatings market has strong need for NMP-free products with similar coating performance to their NMP-containing counterparts. This paper discusses the properties and performance of two Type-2 NMP-free HPDs, and the high performance low VOC coating formulations with judicial selection of coalescing surfactant.
Results and Discussion
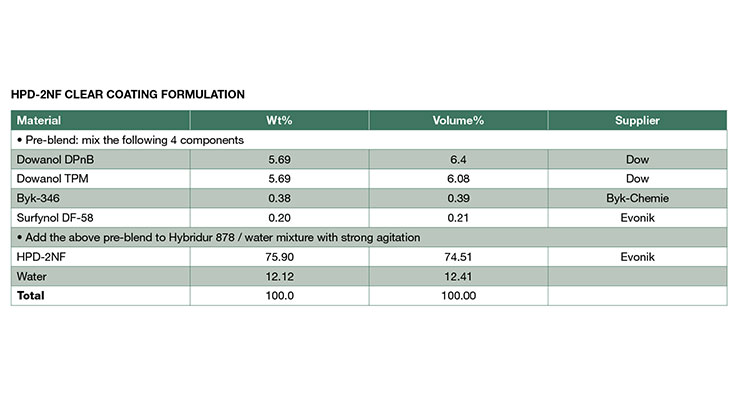
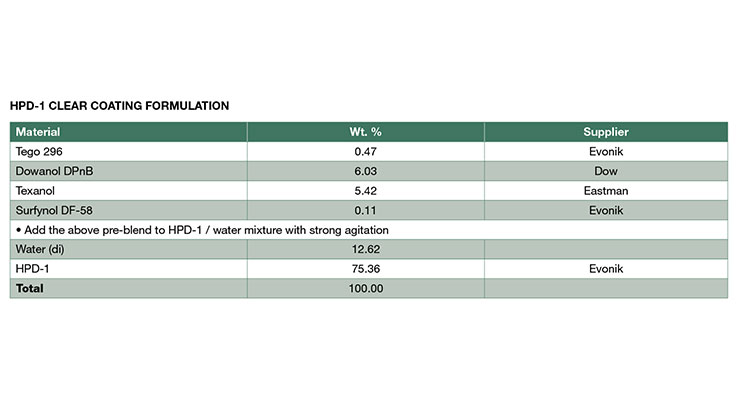
Hybrid Dispersion Characteristics and HPD Polymer PropertiesThe NMP-free HPDs, HPD-1NF and HPD-2NF were prepared and reported according to previously published procedures9-12 and their properties are compared to the analogous NMP-containing HPDs, HPD-1 and HPD-2 in this paper. The composition of the aliphatic urethane portion was identical for all the hybrid polymers and had a glass transition temperature (Tg) about -35 ºC. The acrylic polymer composition was kept similar within the 1-series hybrid and 2-series. The Tg of the acrylic for 1-series is 40 - 50 ºC, and 100 - 110 ºC for 2-series which formed a higher hardness coating. The amount of either urethane or acrylic was about 50% for each HPD. In addition, all the hybrids have similar acid numbers and degrees of neutralization with a tertiary amine. The HPD-1NF was designed for more flexible coating application such as metal and concrete, and the HPD-2NF was developed for very high hardness applications for example plastic film coatings and wood coatings. The two NMP-free hybrids exhibit excellent compatibility and can be blended to tailor desired properties.
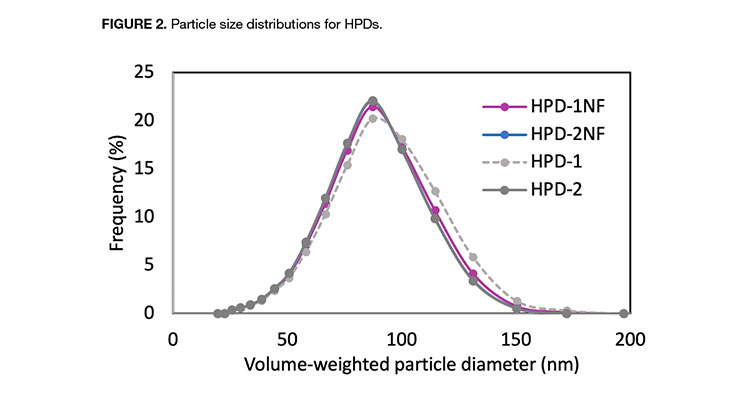
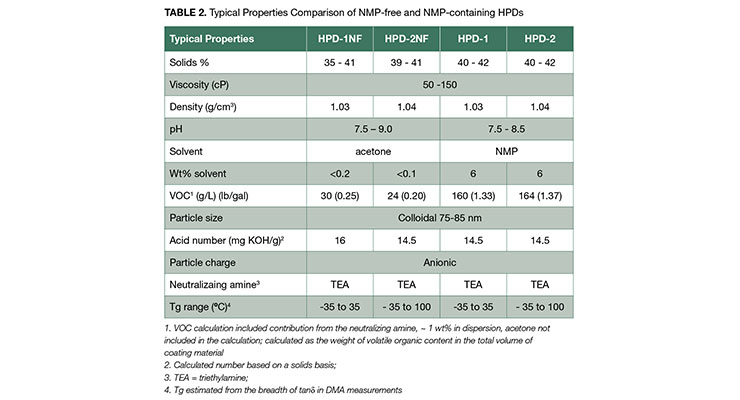
As illustrated in Table 2, the physical property data are very similar for all the HPDs in this paper. Both the NMP-free and NMP-containing hybrids exhibited similar viscosities at the same solid levels. The particle size distributions (Figure 2) for the NMP-free dispersions were similar to those of NMP-containing analogs, and all of the distributions were mono-modal with volume-average particle diameters of 79, 78, 81, and 77 for HPD-1NF, HPD-2NF, HPD-1, and HPD-2 respectively. This indicates that particle size is determined by zeta potential since all of the hybrids have similar acid numbers and degrees of neutralization.13 The particle size data suggests that colloidal NMP-free HPDs can be prepared with proper design of polymer composition.
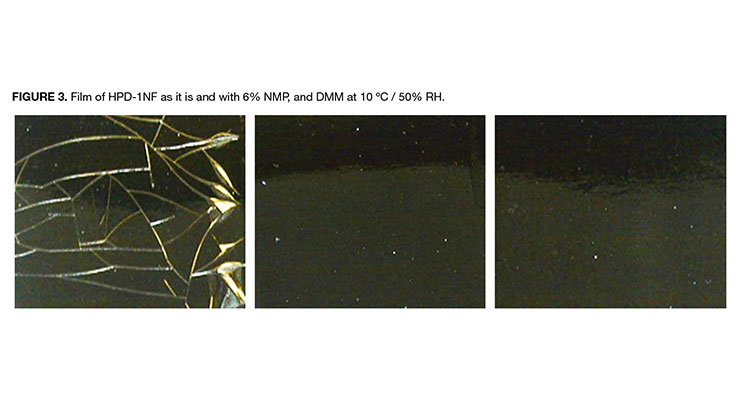
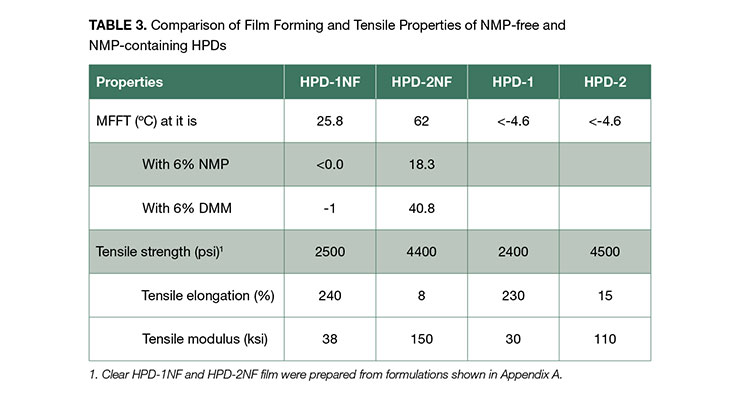
The coating performance of the HPDs largely depends on the chemical composition and the film forming characteristics of the dispersions. Film forming property is principally influenced by how well the particles in HPDs coalesces as water evaporates from coating. The minimum film forming temperature (MFFT) is an effective indicator whether the particles will coalescence at the coating temperature. As demonstrated in Table 3, the NMP-free dispersion HPD-1NF has a MFFT close to room temperature, while HPD-2NF has much higher MFFT. This is consistent with the observation that HPD-1NF formed a clear, but non-continuous (cracked) film shown in Figure 3, whereas HPD-2NF formed a white, flaky film, indicative of poor coalescence.
Addition of 6% of NMP or DMM (dipropylene glycol dimethyl ether) to HPD-1NF and HPD-2NF significantly reduced the MFFT and led to clear HPD-1NF coatings as shown in Figure 3. In contrast, the NMP-containing dispersions HPD-1 and HPD-2 formed clear films because of the presence of NMP in the dispersions.
There is strong regulatory demand to reduce VOC in paints and coatings to be less than 100 g/L in some applications such as stains, waterproofing wood and concrete, and quick dry primer, and less than 50 g/L in other applications for example floor, quick dry enamel and dry fog to be in compliance with the VOC limited by South Coast air Quality Management District (SCAQMD). The data in Table 2 lists the calculated VOC for HPD-1 and HPD-2, both having about 6% of NMP, about 160 g/L. Therefore, they can’t be used to formulate coatings to meet VOC requirement of less than 100 g/L. And for HPD-1NF and HPD-2NF, to meet the VOC requirement of less than 100 g/L, by calculation the amount of added coalescing solvent is limited to less than 3 parts to 100 parts of NMP-free HPD. As shown in the pictures in Figure 3, HPD-1NF required 6% of solvent to form clear coatings. How to achieve low VOC requirement of less than 100 g/L while maintaining the high coating performance will be discussed later in this paper.
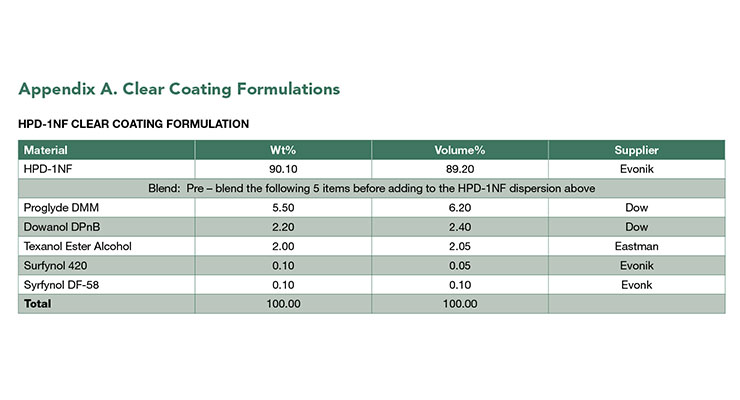
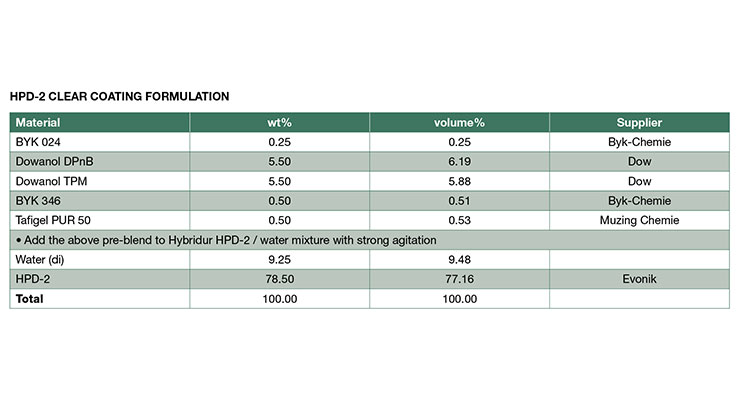
The mechanical properties of clear coatings from NMP-free HPDs were determined by tensile and dynamic mechanical testing and compared to NMP-containing HPDs. The formulations for clear coatings are shown in Appendix A. As shown in Table 3,
The HPD-1NF and HPD-1 have similar modulus and tensile elongation which is much higher than those of HPD-2NF and HPD-2 due to the softer nature of the acrylic portion in the 1-series polymers. The difference in the acrylic portion of HPD-1NF and HPD-2NF is also reflected in the lower Tg as demonstrated in DMA analysis. The 1-series polymers showed a good balance of properties with high elongations (> 230%) and tensile strengths.
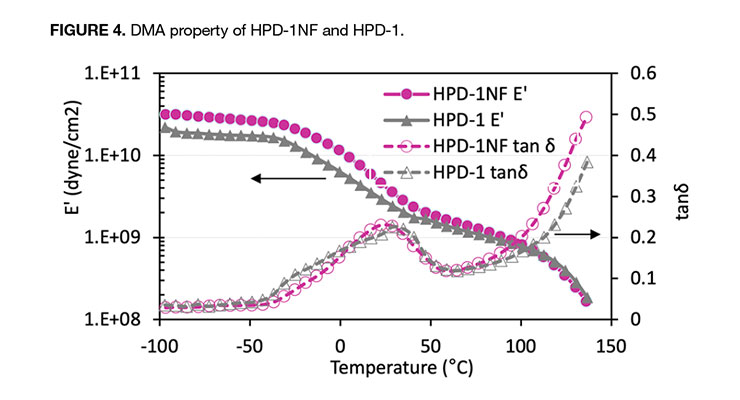
The DMA analysis in Figure 4 compares the dynamic mechanical properties (storage modulus, E’, and tanδ =E”[loss modulus]/E’) as a function of temperature of HPD-1NF to HPD-1. Both show similar trend of storage modulus, E’, versus temperature.
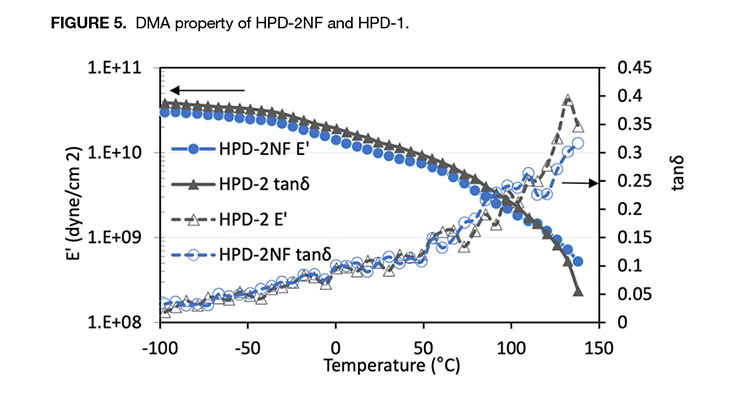
Below Tg of the polyurethane component, the polymer was in the glassy state and had high storage modulus. As temperature increased and near the Tg of acrylic polymer, E’ decreased. Both showed a pronounced rubbery plateau above the Tg of acrylic polymer. The comparison of DMA curves of HPD-2NF and HPD-2 is presented in Figure 5, and both HPDs have very similar E’ and tanδ response to temperature as observed in 1-series HPDs. Due to its high Tg acrylic polymer component, the E’ steadily decreased with temperature, but did not exhibit a rubbery region as shown in 1-series HPDs, and it also took much higher temperature to reach the same E’. The DMA analysis clearly indicates that the NMP-free HPD-1NF has essentially the same mechanical property as the NMP-containing analog HPD-1. The DMA data, in combination with tensile property and the particle size data further confirms that through proper design of polymer composition, NMP-free HPDs can be produced while maintaining similar property to the NMP-containing analogs.
Clear and pigmented coating performance
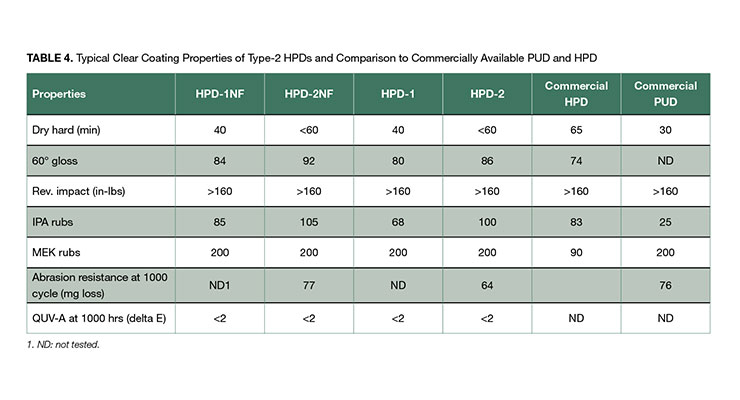
The clear coating performance of the NMP-free HPDs are shown in Table 4, compared to NMP-containing analogs and benchmarked against a commercial NMP-containing PUDs, and an NMP-free HPD. Coating properties for the NMP-free HPD-1NF was similar to those of HPD-1, and HPD-2NF similar to HPD-2 in fast dry speed, gloss, reverse impact resistance, UV resistance, and IPA and MEK resistance. And all compared favorably to the benchmarked commercial materials. All coatings showed a weakness in IPA resistance which can be improved by crosslinking with a proper crosslinker. This will be discussed later in this paper.
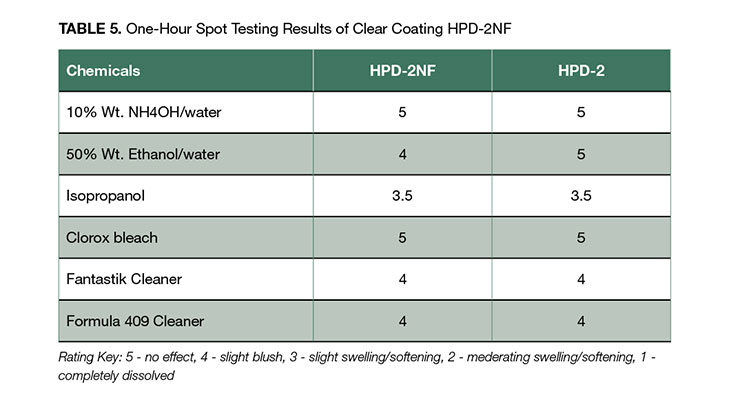
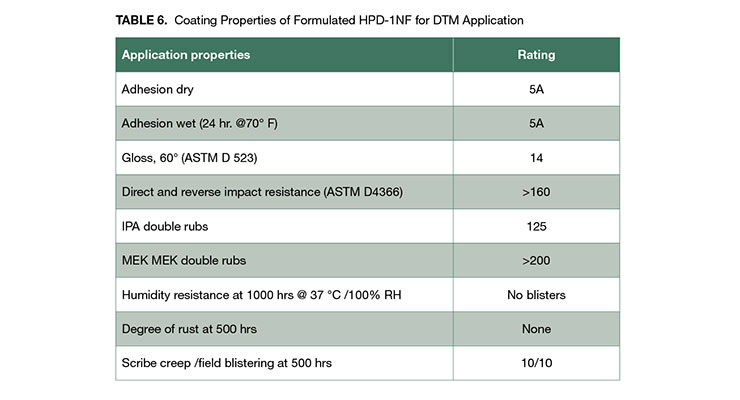
The clear coatings of HPD-1NF and HPD-2NF can be used for interior and exterior applications owing to its excellent adhesion to various substrates, good balance of mechanical property and chemical resistance, and high UV stability. For example, the higher hardness coating of HPD-2NF clear formulation shown in Appendix A was used as coating for clear hard wood floor coating attributable to its hardness and toughness, good wear and abrasion resistance, and excellent chemical resistance shown in Table 5. It has abrasion resistance of weight loss of 77 mg after 1000 cycles, comparable to commercial crosslinked PUD floor coatings of 76 mg.
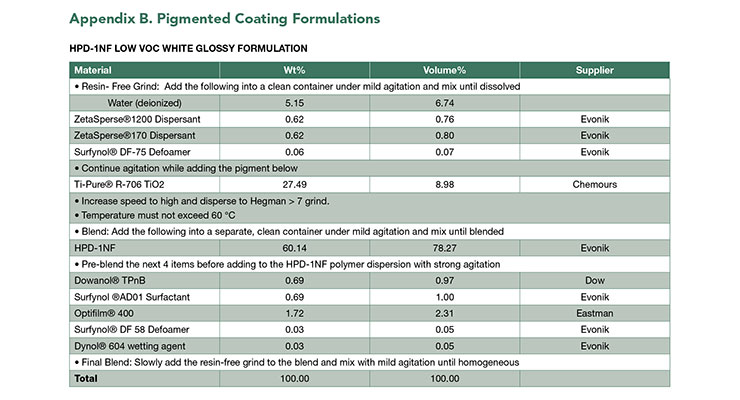
The more flexible and softer coating from HPD-1NF can be directly applied to concrete, with bond strength over 1000 psi. Pigmented HPD-1NF also demonstrated excellent corrosion resistance and can be used for direct to metal (DTM) application. The DTM formulation is shown in Appendix B. The DTM coating has excellent adhesion, good chemical resistance and corrosion resistance as demonstrated in Table 6.
Low VOC formulations with Coalescing Surfactant
Effect of Coalescing Surfactant on MMF
As stated previously, the calculated VOC (calculated as the weight of volatile organic content in the total volume of dry coating, not the wet formulation) of the NMP-containing HPDs is about 160 g/L at typical NMP levels of about 6%. Those polymer dispersions can not be formulated into coatings with a VOC less than 100 g/L. The VOC of neat NMP-free HPD-1NF and HPD-2NF is 30 and 24 g/L respectively, that includes contribution from the neutralizing amine. However, HPD-1NF formed a non-continuous and cracked film by itself as evident in Figure 3, therefore it requires a certain amount of solvent such as NMP to lower the MFFT for particles to coalescence to form a clear coating. By calculation, including the VOC of HPD-1NF, the VOC is calculated to be 93 g/L with 2.5 parts of solvent, and 120 g/L with 3.7 parts of solvent. To achieve a 50 g/L VOC, only about 0.7 parts of solvent should be added. Such low levels of solvent will not be able to lower the minimum film forming temperature enough for particles to coalescence sufficiently. Inadequate coalescence leads to poor film formation thus resulting in inferior coating performance. Satisfactory coating performance such as hardness, gloss, and impact strength, is a result of good coalescence of particles.
It is known that some surfactants can reduce the minimum film-forming temperature (MFFT), therefore improving coalescence, and can be incorporated into coating formulations to impart low to no VOC. Such coalescing surfactants can significantly reduce surface tension and MFFT. In addition, they have solubility parameters similar to those of the polymer binders, and impact minimal effect on formulation and coating properties. The Gemini-type surfactants are particularly effective as coalescing surfactants as they have 2 anchor points and have a greater tendency to remain adsorbed on the particle surface, where they act to plasticize the surface for better particle coalescence.14 This translates to reduce MMF at much lower use level. Also, they do not substantially increase the total VOC since they do not contain solvents.
A Gemini alkane diol surfactant Surfynol AD01 was selected for low VOC formulation. Since there might be small additional contributions to VOC from surfactants and other additives, 2.5 parts of solvent were used in this study to allow a small margin for error to achieve VOC less than 100 g/L. Extensive study was carried out to select proper solvent for optimal coating performance.15 The more hydrophobic and low water solubility solvent Dowanol TPnB (tripropylene glycol n-buty ether) and DPnB (dipropylene glycol n-butyl ether) were chosen for the study since they provided coatings with good water resistance and Persoz hardness.
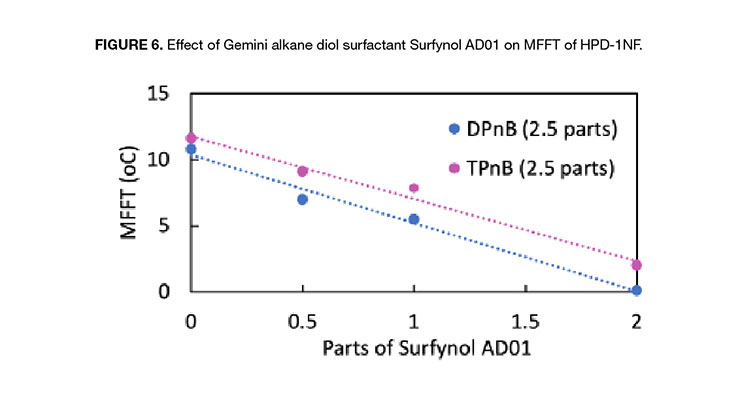
Figure 6 illustrates the effect of Gemini-type surfactant Surfynol AD01 on the MFFT of HPD-1NF where various amount of the surfactant was added to 100 parts of HPD-1NF + 2.5 parts of the selected solvent. The small amount of Gemini alkane diol surfactant reduced the MFFT of the test formulations significantly. The MFFT of neat HPD-1NF was 25.8 ºC, and it dropped to around 11 ºC with 2.5 parts of DPnB, and to 12 ºC with TPnB. Adding 2 parts of surfactant Surfynol AD01 reduced the MFFT substantially to <2 ºC in both formulations.
Clear coating property of formulation with VOC less than 100 g/L
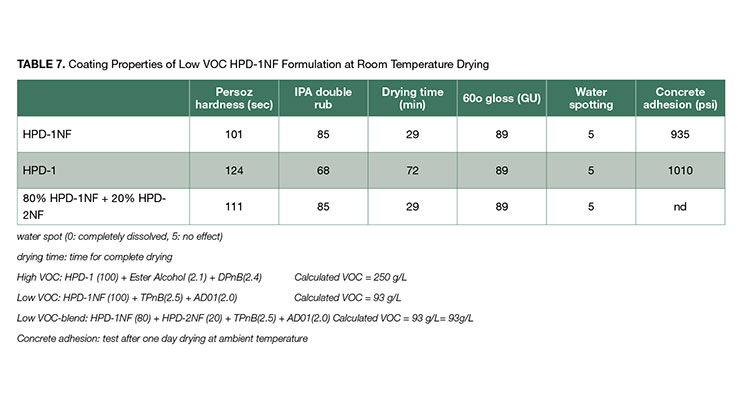
Based on the results above, a low-VOC coating test formulation consisting of HPD-1NF (100) + TPnB (2.5) + Surfynol AD01 (2.0) was used for further testing. The VOC of this test formulation is about 93 g/L, allowing room to use small amounts of other volatile additives and remain below the VOC limit of 100 g/L. The coating properties of low VOC formulation of HPD-1NF was compared with standard high-VOC formulation based on HPD-1 having a VOC of 250 g/L. The coating performance comparison is shown in Table 7.
As shown in Table 7, the low-VOC formulation has equivalent properties in water spot resistance, 60º gloss and concrete adhesion. IPA double rubs was improved in the low VOC HPD-1NF formulation. The complete drying time of the low VOC HPD-1NF coating was 29 minutes, which was faster than the high VOC HPD-1 formulation of 72 minutes due to the relatively slow evaporation rate of NMP in HPD-1. On the other hand, the Persoz hardness of 101 seconds for the low-VOC HPD-1NF coating, was a little lower than that of high-VOC of 124 seconds, due to slight Tg depression by the Gemini surfactant Surfynol AD01. Coatings exhibited a weakness to IPA resistance as reflected in low numbers of IPA double rub test.
The low Persoz hardness of the low-VOC HPD-1NF formulation can be improved by blending with the high-Tg HPD-2NF. As described in Table 2, the HPD-2NF has a Tg range of -35 ~ 100 ºC so that its hardness is much higher than HPD-1NF. It is expected that the blend of HPD-1NF and HPD-2NF can improve the Persoz hardness of the coating. The coating properties of the blend are shown in Table 7 where 20% of HPD-1NF was replaced with high-Tg HPD-2NF. As expected, the Persoz hardness increases from 101 to 111 seconds while all other properties remained unchanged including IPA double rub. The hardness can be further increased with higher level of HPD-2NF, however, more solvent will be needed to maintain a satisfactory level of coalescence.
Crosslinking and heating to enhance clear coating properties
Coating properties especially chemical resistance can be improved by heating, crosslinking, or the combination of both. The HPDs described in this paper contain carboxylic acid groups, which serve as a convenient functional group to be crosslinked. A number of crosslinking chemistries are known to crosslink with carboxylic acid group in PUDs and HPDs, for example, polyaziridines, carbodiimides, water dispersible isocyanates and epoxies, metal salts such as zinc and zirconium, and silanes such as beta-(3,4- epoxycyclohexyl)ethyltriethoxysilane (a cycloaliphatic epoxy-silane) and 3-glycidoxypropyltrimethoxysilan. Most of these chemistries are for 2K system, only beta-(3,4- epoxycyclohexyl)ethyltriethoxysilane was found to be suitable for 1K system.
It has been reported that chemical resistance properties of coatings based on NMP-containing HPDs was improved via crosslinking with beta-(3,4- epoxycyclohexyl) ethyltriethoxysilane (a cycloaliphatic epoxy-silane)15, 16 and can be incorporated into formulations to develop a shelf-stable (at least 6 months) high-VOC HPD 1K formulations.
Heating is another approach to improve chemical resistance. Waterborne dispersions are used both for room temperature curing and heat cure processes, for example, a coated part is passed through a drying chamber for a short duration of time during production. The advantage of a heat cured system is high speed, but coating performance can suffer compared to cure at room temperature for several days, depending on the exact heat-cure conditions such as line speed and oven temperature.
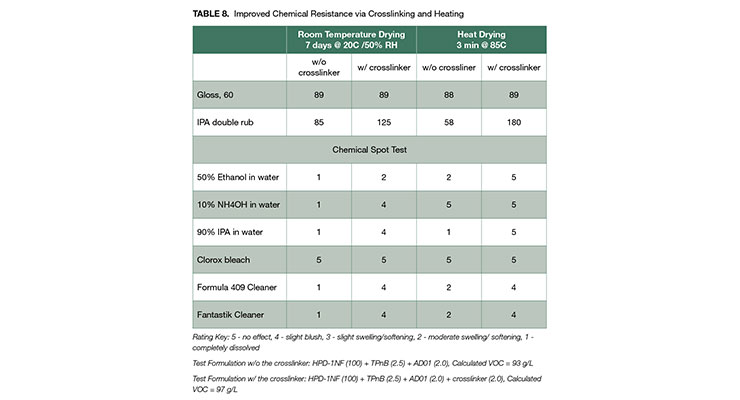
Table 8 illustrates the chemical resistance enhancement of the low VOC formulations based on HPD-1NF with beta-(3,4- epoxycyclohexyl)ethyltriethoxysilane crosslinker at room temperature cure and fast heat cure conditions. Heat curing was conducted by placing coated specimens in an oven at 185 ºF (85 ºC) for 3 minutes.
As noted in this paper, one of the weakness of HPDs and PUDs is IPA resistance. Data in in Table 8 clearly shows the drastically improved IPA double rub property for both room temperature and heat cured coatings. The IPA double rub resistance of the heat cured formulation containing the crosslinker was especially notable, which was previously only achieved in high VOC HPD-1 coatings using the same crosslinker. It is important to note that this high level of IPA resistance is now being achieved at a VOC of less than 100 g/L. Resistance to a broad range of chemicals is often required for coating applications. Spot test results for 6 different chemicals are also summarized in Table 8. Chemical resistance was improved through crosslinking with an epoxy-silane crosslinker, and was further improved through heat cure.
Low VOC pigmented coating performance
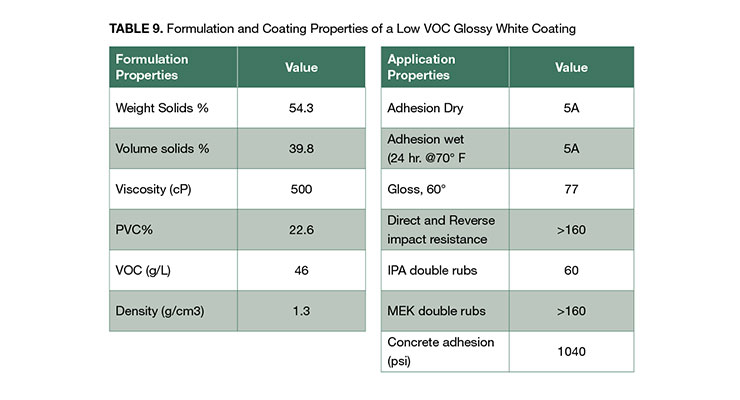
The concept of using coalescing surfactant to achieve high performance low VOC in clear coatings can be applied to pigmented formulations. A white glossy formulation based on HPD-1NF with VOC less than 46 g/L was developed. The coating has good impact and chemical resistance, and excellent dry and wet adhesion to metal substrate and concrete. Concrete adhesion was tested after 1 day of drying at room temperature. Detailed formulation is shown in Appendix B.
Experimental
NMP free HPDs (HPD-1NF and HPD-2NF) and NMP containing (HPD-1 and HPD-2) were prepared according to the procedures outlined previously.10, 11 Coating formulations (Appendix A and B) were prepared using standard techniques. Coating properties were tested over cold-rolled steel with a zinc phosphate treatment (Bonderite 952), untreated cold-rolled steel, or on sealed-paper charts (Leneta Corporation). The coatings were applied using a #60 wire-wound drawdown rod and were typically allowed to dry at 21 ºC and 50% relative humidity for seven days unless otherwise specified. Depending on the formulation, the dried film thickness ranged from 30 micron (1.2 mil) to 76 micron (3.0 mil).
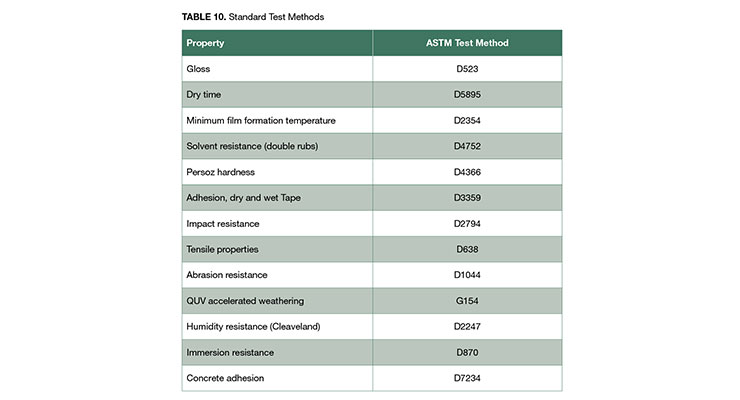
The standard test methods listed in Table 10 were used to evaluate coating performance. Spot tests were performed on clear coatings applied by drawdown on sealed-paper charts. The coatings were dried for 24 hours at 21 ºC and 50% relative humidity and the spots (2-3 cm wide) were rated after exposure to each reagent for one hour. The reagent spots were covered with a watch glass during the exposure to prevent evaporation. Prior to evaluating the coating, the reagent spots were removed by lightly patting with a clean paper towel.
DMA data was obtained on clear resin coatings (Appendix A) using a Rheometrics Solids Analyzer RSAII (Rheometric Scientific) in a tensile dynamic mode with a thin film fixture. The films were analyzed over the temperature range from -150 ºC to 150 ºC.
The samples were not preconditioned with regard to humidity prior to data acquisition, but dry nitrogen was used as the atmosphere during the measurements. Data were acquired at intervals of 6 ºC; a one-minute soak time was used at each measurement temperature to ensure isothermal equilibration. MFFT results were obtained using a Minimum Film Formation Temperature Bar Model MFFT-90 (Rhopoint Instrumentation Ltd.). Films were applied by drawdown to a wet film thickness of 152 micron (6 mils). Tensile data were obtained on clear films that had an average thickness of ~152 micron (6 mils) and were dried at 21 ºC and 50% relative humidity (RH) for seven days. The crosshead speed used was 2 inch/min and the temperature was 23 ºC with 50% RH. Particle size determinations were made using an LA-910 Laser Scattering Particle Size Distribution Analyzer (Horiba).
Summary and Conclusions
High performance waterborne NMP-free urethane-acrylic hybrid dispersions (HPD), HPD-1NF and HPD-2NF have been developed to offer cost/performance advantages over standard 1K coating materials such as polyurethane dispersions (PUDs). They were designed to meet the market needs for safer products and increasingly stringent low VOC requirements. The HPD-1NF has a lower Tg acrylic component and is more flexible and softer while the HPD-2NF has a higher Tg acrylics and is tougher and harder. These two HPDs are fully compatible with each other and can be blended to achieve tailored properties.
The HPDs were prepared by an in-situ process where the urethane and acrylic were polymerized together as a homogenous mixture after being dispersed as colloidal particles in water. The unique process resulted in an IPN-like structure apparently responsible for the hybrid’s outstanding mechanical and chemical resistance properties. The NMP-free HPDs have been shown to provide dispersion (e.g. particle size, viscosity, pH) and coating properties (e.g. Tg, tensile strength, chemical resistance, drying time, impact resistance) comparable to their NMP-containing counterparts. The data suggests that colloidal NMP-free HPDs can be prepared with proper design of polymer composition without the need to add NMP during process.
These NMP-free HPDs provide coatings with rapid dry, fast property development, good adhesion, excellent flexibility and toughness, good hardness, and excellent chemical resistance. They can be used in high performance coating applications, for example HPD-2NF can be used as wood floor coatings where good chemical resistance, abrasion/scratch resistance, and excellent UV stability are targeted attributes, and HPD-1NF for direct to metal applications where flexibility, adhesion and corrosion resistance and barrier property are critical.
Due to the NMP-free nature of the HPDs, it is possible to develop coating formulations with high performance while meeting the low VOC requirement of less than 100 g/L. A Gemini alkane diol type surfactant Surfynol AD01 was found to be an effective coalescing surfactant and capable of reduce MFFT at much lower use level than typical coalescing solvents. This surfactant and tripropylene glycol n-butyl ether (TPnB) were selected in clear coating model study to demonstrate low VOC feasibility. Clear and pigmented coating formulations meeting the less than 100 g/L VOC target were developed and the resulting coatings exhibited comparable excellent properties compared to those from much higher VOC coating formulations. The chemical resistance of coatings from the NMP-free HPD-1NF was further enhanced via the use of an epoxy-silane crosslinker and heat curing at 85 ºC for 3 minutes.
Acknowledgements
The authors would like to thank all the people who have contributed over the years to the development of the HPD technology. The authors wish to extend their gratitude specially to the following for their contribution: former Air Products & Chemicals Inc. employees Richard Derby, Bruce Gruber, Menas Vratsanos, Dennis Nagy, Ernest Galgoci, Charles Hegedus, and Frederick Walker for their technical work, reports, articles, and presentations incorporated in this paper.
References
1. Lambourne, R., Paint and Surface Coatings: Theory and Practice, John Wiley and Sons, New York, 1987.
2. Dieterich, D., Prog. Org. Coat., 9,281 (1981)
3. P. Wright and A. Cumming, Solid Polyurethane Elastomers. MacLaren and Sons, London, 1969.
4. Z. S. Petrovic and J. Ferguson J, Prog. Polym. Sci, 1991, 16, 695–836.
5. Z. S. Petrovic, Polyurethanes, in Handbook of Polymer Synthesis, ed. by H. R. Kriecheldorf, O. Nuyken and G. Swift, Marcel Dekker, New York, 2005, 503–540.
6. L. E. Nielsen, Mechanical Properties of Polymers and Composites; Volume 1 and 2, Marcel Dekker,New York, 1974
7. C. R. Hegedus and K. A. Kloiber, Journal of Coatings Technology, 1996, 68 (860), 39-48.
8. H. L. Honig, C. Suling, D. Dieterich and A. Reischl, U.S. Patent 3,684,758, 1972.
9. HPD-1NF: Hybridur 870, HPD-2NF: Hybridur 878, HPD-1: Hybridur 570, and HPE-2: Hybridur 580. Further details are included in the respective technical datasheets from Evonik Corporation.
10. P. Loewrigkeit and K. A. Van Dyk, U.S. Patent 4,644,030, 1987.
11. B. R. Vijayendran, R. Derby and B. A. Brube, U.S. Patent, 5,173,526, 1992.
12. E. C. Galgoci, C. R. Hegedus, F. H. Walker, D. J. Tempel, F. R. Pepe, K. A. Yoxheimer and A. S. Boyce, J. Ctg. Tech. 2005, 2 (2), 28-36.
13. Y. Chen and Y. - L. Chen, J. Appl. Polym. Sci., 1992, 46, 435-443.
14. E. C. Galgoci, K. Yacoub, V. V. Shah, R. Reinartz, K. A. Yoxheimer and S. Y. Chan, J. Ctg Tech. CoatingsTech, 2006, 3(1), 34–42.
15. T. J. Lim, E. C. Galgoci, F. H. Walker and K. A. Yoxheimer, Eur. Ctg J. 2005, 5, 24-30.
16. M. J. Chen, J. Ctg Tech. 1997, 69 (875), 49-55.