09.28.16
A bright blue compound that was first discovered by accident seven years ago in an Oregon State University laboratory – and has since garnered global attention – has now led to the more rational and methodical development of other colors that may ultimately change the world of pigments.
Findings on the newest pigments, in shades of violet and purple, were just published in Inorganic Chemistry, a journal of the American Chemical Society.
More important, researchers say, is that progress made since the first accidental discovery of this family of inorganic compounds has allowed intensive science to take the place of luck. What’s emerging is a fundamental understanding of the chemistry involved in these “trigonal bipyramidal” compounds.
As the basis for pigments, they are quite remarkable.
Compared to the flaws that exist in many of the compounds they replace, they are all thermally stable, chemically inert, non-toxic and non-carcinogenic. For commercial use, they also have the extraordinary characteristic of reflecting heat, which is highly unusual for dark colors and potentially of great value for saving energy.
All of the compounds have been patented, and are being developed commercially by a private company. Yellow, green and orange colors have already been created, along with the original blue. The research has been supported by the National Science Foundation.
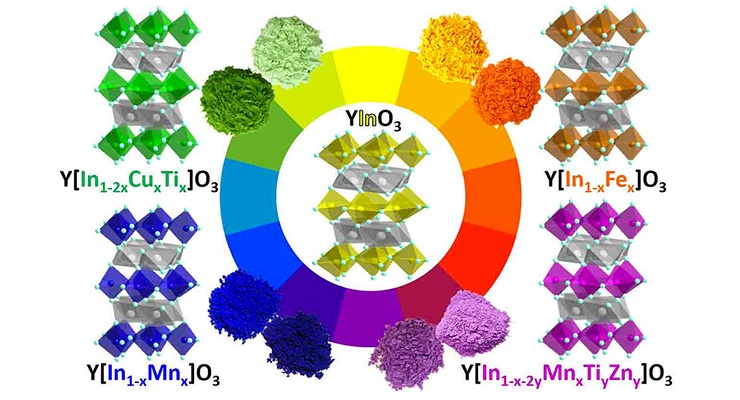
These developments began in 2009 when OSU researchers were studying some manganese oxide compounds for their potential electronic properties, and when one compound came out of an extraordinarily hot oven – about 2,000 degrees Fahrenheit – it had turned a vivid blue, now known as “YInMn” blue.
The scientists noticed and took advantage of this unexpected result. They used the compound to create a pigment that was environmentally benign, resisted heat and acid, and was easily made from readily available raw materials.
“No one knew then that these compounds existed,” said Mas Subramanian, the Milton Harris Professor of Materials Science in the OSU College of Science, and corresponding author on the new publication.
“Now we’ve been able to move beyond the accident and really understand the chemistry, including its structure and synthesis. We can produce different colors by using the same basic chemical structure but tweaking things a little, by replacing manganese atoms by iron, copper, zinc and/or titanium. And we’re slowly moving toward what we really want, what everyone keeps asking for, the Holy Grail of pigments - a bright, new, durable, nontoxic red.”
Along with blue, Subramanian said, a stable, nonorganic red pigment would have huge commercial demand.
In this process, the OSU researchers are opening the door to new, inexpensive types of pigments that leave behind some of the toxic compounds historically used to create colors – lead, cadmium, mercury, even arsenic and cyanide. And the bonus of solar heat reflection has huge value for many applications, such as building construction or vehicles, where this characteristic can reduce cooling expenses and something other than white is desired.
Based on the novelty of the discovery and the growing value of these pigments, this research has captured international media attention and broad public fascination – a single online video received 14 million views.
The newest colors of violet and purple, the researchers noted in their study, have long been associated with royalty, aristocracy, piety and faith. The first pigments of these colors date back to cave paintings in France in 25,000 B.C., they said. And Chinese Han purple, the first synthetic purple pigment, was found in some murals in tombs more than 2,000 years old.
Pigments still being used to produce these colors are in some cases chemically and thermally unstable, and subject to increasing environmental regulations.
Applications of the new pigments, the researchers said in their report, may be found in high-performance plastics and coatings, building exteriors, cool roofing, vinyl siding, automobiles, and even art production or restoration.
Findings on the newest pigments, in shades of violet and purple, were just published in Inorganic Chemistry, a journal of the American Chemical Society.
More important, researchers say, is that progress made since the first accidental discovery of this family of inorganic compounds has allowed intensive science to take the place of luck. What’s emerging is a fundamental understanding of the chemistry involved in these “trigonal bipyramidal” compounds.
As the basis for pigments, they are quite remarkable.
Compared to the flaws that exist in many of the compounds they replace, they are all thermally stable, chemically inert, non-toxic and non-carcinogenic. For commercial use, they also have the extraordinary characteristic of reflecting heat, which is highly unusual for dark colors and potentially of great value for saving energy.
All of the compounds have been patented, and are being developed commercially by a private company. Yellow, green and orange colors have already been created, along with the original blue. The research has been supported by the National Science Foundation.

The scientists noticed and took advantage of this unexpected result. They used the compound to create a pigment that was environmentally benign, resisted heat and acid, and was easily made from readily available raw materials.
“No one knew then that these compounds existed,” said Mas Subramanian, the Milton Harris Professor of Materials Science in the OSU College of Science, and corresponding author on the new publication.
“Now we’ve been able to move beyond the accident and really understand the chemistry, including its structure and synthesis. We can produce different colors by using the same basic chemical structure but tweaking things a little, by replacing manganese atoms by iron, copper, zinc and/or titanium. And we’re slowly moving toward what we really want, what everyone keeps asking for, the Holy Grail of pigments - a bright, new, durable, nontoxic red.”
Along with blue, Subramanian said, a stable, nonorganic red pigment would have huge commercial demand.
In this process, the OSU researchers are opening the door to new, inexpensive types of pigments that leave behind some of the toxic compounds historically used to create colors – lead, cadmium, mercury, even arsenic and cyanide. And the bonus of solar heat reflection has huge value for many applications, such as building construction or vehicles, where this characteristic can reduce cooling expenses and something other than white is desired.
Based on the novelty of the discovery and the growing value of these pigments, this research has captured international media attention and broad public fascination – a single online video received 14 million views.
The newest colors of violet and purple, the researchers noted in their study, have long been associated with royalty, aristocracy, piety and faith. The first pigments of these colors date back to cave paintings in France in 25,000 B.C., they said. And Chinese Han purple, the first synthetic purple pigment, was found in some murals in tombs more than 2,000 years old.
Pigments still being used to produce these colors are in some cases chemically and thermally unstable, and subject to increasing environmental regulations.
Applications of the new pigments, the researchers said in their report, may be found in high-performance plastics and coatings, building exteriors, cool roofing, vinyl siding, automobiles, and even art production or restoration.

















