Brittney M. McInnis, Jonathan D. Hurt, Steve McDaniel, Lisa K. Kemp, Tyler W. Hodges, and David R. Nobles Jr., Reactive Surfaces, The University Of Southern Mississippi, The University Of Alabama, and The University Of Texas07.03.19
Summary
Coatings capable of adhering to surfaces routinely exposed to light can exploit a biomimetic system similar to surfaces covered with photosynthetic lichen in order to capture, fix, and sequester carbon dioxide. When applied over sufficiently large amounts of surface area, such coatings are capable of rivaling natural carbon sinks in their ability to remove excess atmospheric carbon. The coatings industry stands in a unique position to cooperatively and profitably address climate change caused by excess carbon dioxide and to do so in a timely manner geared to preventing the most damaging of predicted environmental harm. By capturing large quantities of such greenhouse gases and sequestering the captured carbon into carbohydrates such as cellulose, these paints are capable of manufacturing useful byproducts, including some of the raw materials from which they themselves are formulated. This is the first technical description of these types of coatings.
Introduction
It is well known that carbon dioxide is a by-product of both naturally occurring and man-made activities. Examples of such naturally occurring activities include: animal respiration, decomposition of formerly living organisms, weathering of carbonate rocks, volcanic eruptions, and plant (e.g., forest) fire; examples of man-made activities (anthropogenic) include: fossil fuel use, intentional burning of biomass (e.g., wood stoves, intentional forest fires), and cement production. However, the current, drastic increase in carbon dioxide in the Earth’s atmosphere is being driven by release of additional carbon dioxide by mostly terrestrial, man-made activities, particularly in cities and industrialized regions. 1,2
Photosynthesis from land-based plants (including lichen and other cryptogamic species) and from ocean-bound algae (including cyanobacteria which are photosynthetic prokaryotes) is the primary, naturally occurring process for removing carbon dioxide from the Earth’s atmosphere. This naturally occurring photosynthetic conversion of carbon dioxide (CO2) requires water (which can be in the form of environmental moisture) and sunlight to convert available carbon dioxide in the atmosphere to oxygen (O2) and carbohydrates (e.g., saccharides). The ocean-bound algae are in the form of a thin layer floating at/near the surface of ocean water, known as the photic zone, and the vast size of the earth’s oceans provides a substantial surface area by which these ocean-bound algae can perform the majority (70 to 80%) of photosynthetic capture of atmospheric carbon dioxide and release of oxygen into the atmosphere. 3,4
However, because of the sheer magnitude of carbon-dioxide resulting from man-made activities, naturally occurring photosynthetic conversion of carbon dioxide has for many decades been unable to mitigate the amount of carbon-dioxide produced by man-made activities. As a result, excess amounts of carbon dioxide have built up in the atmosphere. It is estimated that the amount of carbon dioxide in the Earth’s atmosphere has increased from 280 parts per million (ppm) in the 1700s to 411 ppm as of March 2019.5 To make matters worse, approximately 50 gigatons of additional carbon dioxide equivalents (e.g., greenhouse gases such as carbon dioxide, methane, nitrous oxide, hydrofluorocarbons, perfluorocarbons, sulfur hexafluoride) are released into the Earth’s atmosphere yearly, primarily from fossil fuel usage.6 It is well known that carbon dioxide represents about 80% of “greenhouse gas,” with methane, nitrous oxide, and fluorinated gases representing the balance. It is also well known that these greenhouse gases are the primary contributing factor to global warming and the associated climate change around the globe (i.e., severe storms, warming of the oceans, melting of glaciers, rising sea levels). The current level of greenhouse gases and their projected rate of increase have led experts in the field of climatology to the conclusion that, if left unchecked, further increases in greenhouse gases will result in irreversible climatic changes and catastrophic effects of climate change on people, property, and the ecosystem.1,2
To combat the current level of greenhouse gases and their projected rate of increase, various forms of negative emission technologies are being developed. A negative emission technology removes (i.e., captures) carbon dioxide equivalents from the atmosphere where they may be sequestered or stored for long periods of time (i.e., years, decades, centuries or longer).7
Negative emission technologies can include enhanced carbon sinks that provide for long-term storage of the removed carbon dioxide (and/or other component(s) of greenhouse gas). Examples of enhanced carbon sinks include storage of captured carbon dioxide underneath the surface of the earth, conversion of captured carbon to useful solid or liquid materials such as plastics, carbon fibers, biofuel, or carbon-based chemicals.8,9 Some other negative emission technologies include: geological carbon sequestration, direct carbon dioxide capture from the atmosphere, bioenergy carbon capture and storage, coastal blue carbon capture, terrestrial carbon sequestration, and carbon mineralization of carbon dioxide.10
In much the same way that the magnitude of photosynthetic conversion of atmospheric carbon dioxide by ocean-bound algae is limited by the surface area of the ocean, known negative emissions technologies are limited in their efficiency and effectiveness for mitigating carbon dioxide by their available footprint (terrestrial or otherwise). Surface areas occupied by facilities housing these negative emissions technologies must do so in a manner that is practically feasible from societal and financial perspectives.11,12 Therefore, a negative emissions technological solution that simulates the unequalled efficiency and effectiveness of ocean-bound algae, but in a manner that provides for markedly greater surface area-to-volume practicalities, would be advantageous, desirable, and useful.
The coatings industry is a world-wide industry servicing paintable-surface-area measured in the billions of square meters, much if not most of which coated area is vertically oriented or otherwise incorporated into vertical structures such as buildings, glass windows, plastic components, etc. When coupled with myriad other types of articles of man-made manufacturing, the actual surface area coated yearly is staggering. Even more enormous is the true surface area of a porous coating itself, which cannot be seen by eye.13 To mimic this almost unlimited amount of vertical surface area to test the ability of carbon capture coatings to drawdown sizeable portions of anthropogenic carbon dioxide, an economical model system comprising translucent plastic sleeves containing translucent PET containers with easily measurable interior paintable surface area was constructed. While this model does not entirely mimic all surfaces amenable to carbon capture coating (CCC) coverage, it allows the construction of massively iterated vertical surface (MIVS) devices (CCC-MIVS) that may be useful to initiate a global-scale coating effort that can immediately be placed into service drawing down large amounts of CO2.
Experimentsm and Results
From the Ocean to a Flask
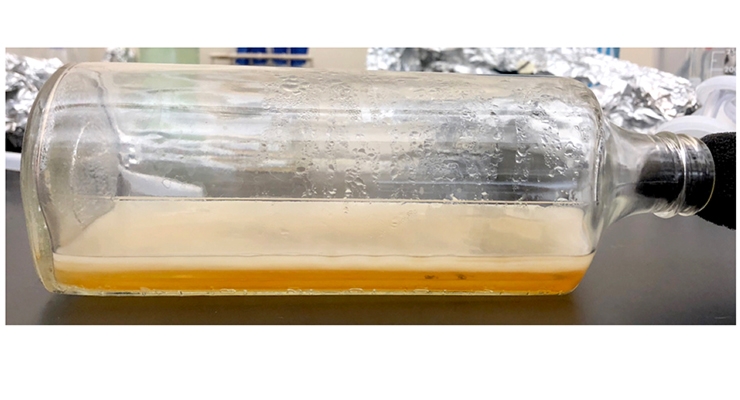
Culturing algae has long been performed with great success, and our collaboration with the Culture Collection of Algae at the University of Texas at Austin (UTEX; one of the most extensive collections of algae in the world) has provided us with access to thousands of potential algae candidates. Here we report our work with a particular algal (cyanobacterial) strain, Synechococcus leopoliensis (UTEX strain 2434, “wild type”). These algae have also been genetically engineered to produce additional cellulose (NS::abΔc7S, “overproducer”)14, and we examined both the wild type and the engineered cellulose overproducer in this work. Figure 1 shows both cultures being grown in glass jars under LED grow lights in our laboratory.
Out of the Flask and Almost a “Coating”
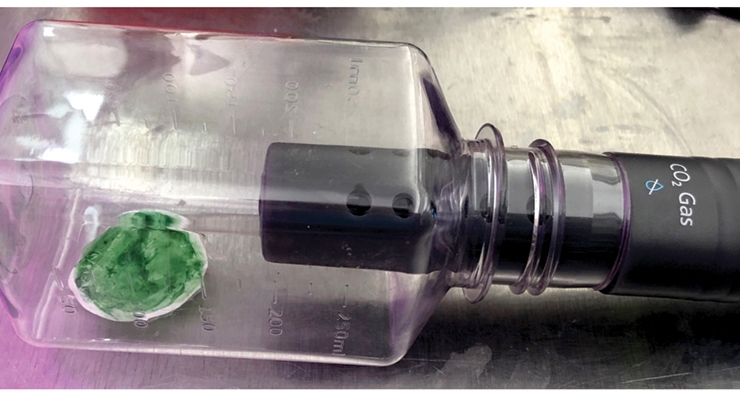
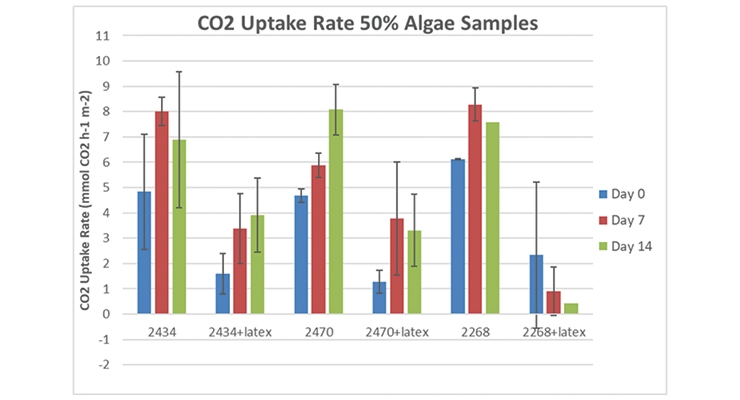
Bernal et al.15 reported the use of algae mixed 1:1 with a latex resin and coated onto paper in an effort to make a “synthetic cellular biocomposite leaf.” They reported CO2 uptake rates of up to 5.67 mmol CO2 hr-1 m-2 for cells kept hydrated by wicking an algae culture medium onto the coated filter paper in an artificially increased CO2 environment (20% CO2). These researchers stated their belief that similar systems should be capable of exceeding the rates of carbon capture seen in green plants such as Arabidopsis (18 mmol CO2 hr-1 m-2), and we agree. We chose to replicate their system using several test algae without the artificial CO2 environment to see if our rates of CO2 uptake would be similar. Figure 2 shows a typical filter paper sample containing either algae cells alone or a 1:1 wet mixture of algae cells and latex resin being measured for its CO2 uptake rate. The filters were kept moist by placing them on fresh agar pucks. Our CO2 uptake rates were very similar to those of Bernal et al. when tested for an hour of 250 PAR LED light exposure. These samples were followed with time, and the CO2 uptake rates are shown in Figure 3.
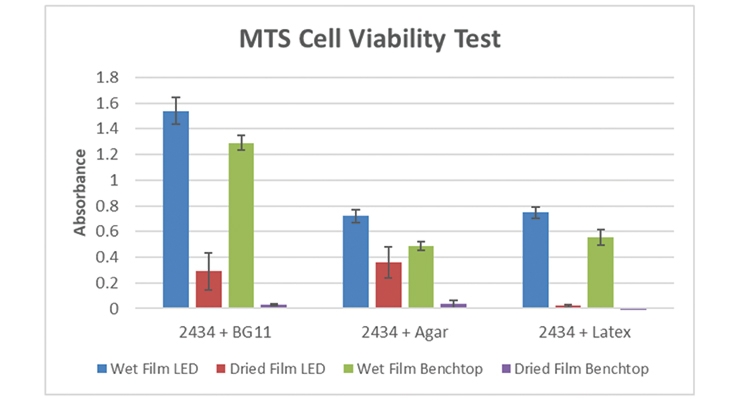
Once we had evidence of CO2 uptake for both the cells alone and the cells mixed with latex resin, we tested the viability of the algae cells mixed with BG-11 media, agar, or an acrylic latex resin as either dried films or in the wet state. We also tested the samples under different lighting conditions: under LED illumination or under ambient laboratory light. Each sample was run in triplicate, and controls for the BG-11, agar, and latex resin were included. Figure 4 shows the test plate after the MTS viability testing where a darker red/brown color indicates increased cell metabolism, which correlates with positive cell health and/or growth. Alcohol killed samples and heat killed samples were used as negative controls and showed no reaction in the test. Figure 5 shows the numerical comparison for the testing of the cells alone and with the polymer systems. In all cases, the wet films had higher performance (in some cases much higher performance) than the dry films. This led us to develop a highly-hydrated coating that did not require constant wicking of water or medium and that would better support the viability and CO2 uptake of the algae over long periods of time.
Super-Hydrated Algae Gel-Coatings
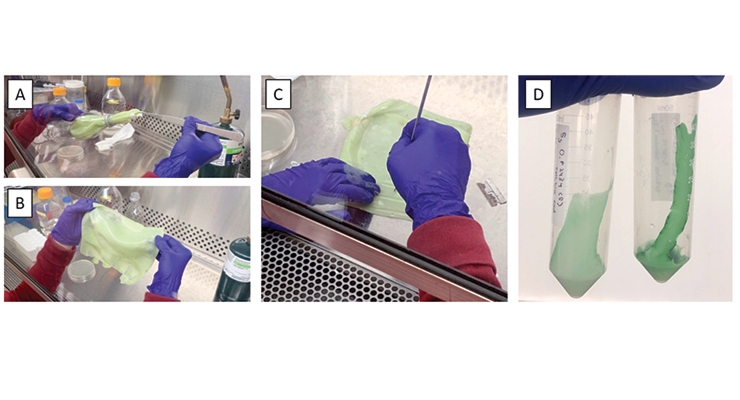
Based on the results in the MTS viability studies and our review of literature on lichens (that perform this function in nature),16 it was determined that high levels of hydration in the coating (80-100%) could lead to improved viability of the cells and increased photosynthesis. It was our goal to develop a coating based on natural materials from renewable resources, and we screened many water absorbing polymers. The system that best met our requirements was a blended system of sodium alginate (we chose a commercial, pre-formulated alginate) and xanthan gum. This system has many advantages: it can absorb many times its weight in water, is tolerant to moderate levels of ions and minerals (supplied in the media as nutrients for the algae), and gels into a pliable, semi-solid that can retain its shape on a vertically positioned surface – essentially a polymeric, hydrogel coating. The algae cells are dispersed in the media or water used to hydrate the coating, and upon the addition of the liquid, the alginate begins the crosslinking process with about 5-8 minutes of total working time before the material sets into a semi-solid state. Figure 6 shows the process of making the coating mixture and applying it to the PET containers.
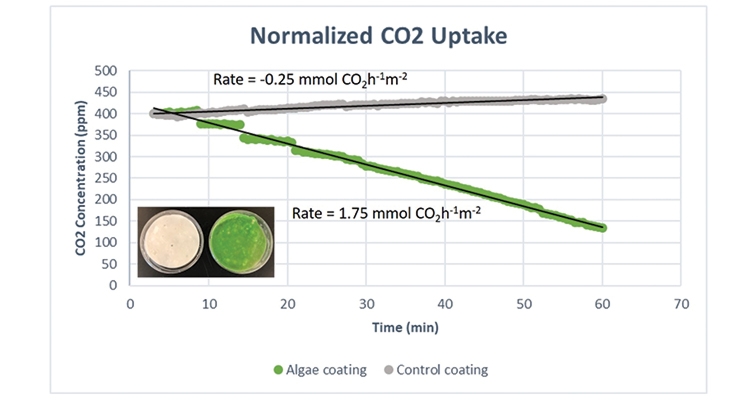
Once the PET containers are coated with the algae-containing carbon capture coating, they can either be measured as single devices or placed into plastic sleeves and measured as a group of samples (Figure 7). The CO2 uptake rates are compared in Figure 8 showing the change in CO2 with time for a flat plastic dish containing the coating without algae and for one with algae. The rate over the one-hour measurement period for the control was slightly negative (CO2 released, no uptake), but the rate was 1.75 mmol CO2 per m2 of coating surface area (rate reported as mmol CO2 h-1 m-2) for the sample containing 2.5% algae.
Evidence of Algae Growth and Longevity in Gel-Coatings
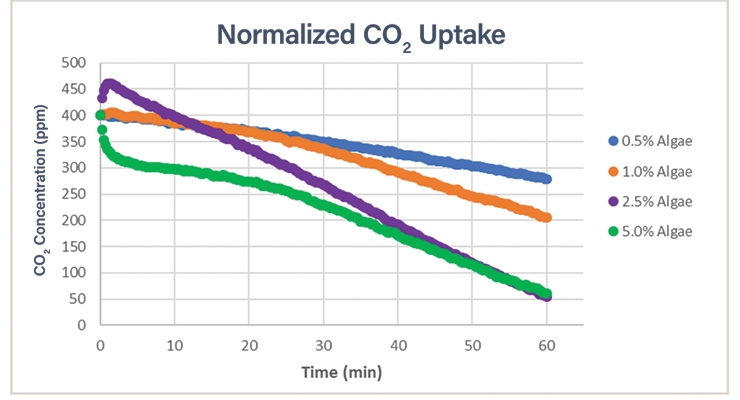
Several formulations were tested on the PET substrates to understand CO2 capture rates of the coatings. As an example, we studied the algae level initially added to the coating to understand what level might provide optimal CO2 uptake and were pleased to see that the algae have an extended lifetime in this coating system. Pictured in Figure 9 is an array of sleeves of the coated PET containers, which show the samples on the day they were made (day 0), as well as on days 10 and 45 after the samples were made. From left to right in the photo, the samples contain increasing amounts of algae cells: 0.5%, 1.0%, 2.5%, and 5.0%.
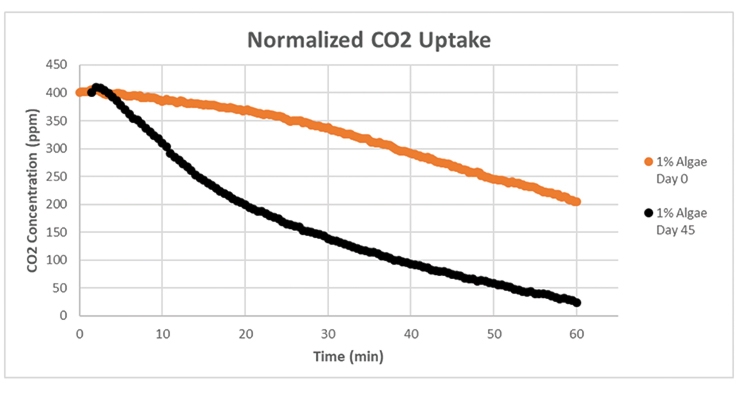
As expected, the green color in the PET containers darkens as the amount of algae in the PET container increases. The CO2 uptake rates on day 0 (normalized to the same starting concentration in Figure 10), which were measured for each sleeve of PET containers (5 PET containers total), also shows an increase with the increasing amount of algae until the rate equalizes between the samples at 2.5% and 5% algae concentration. As the coated PET containers age, the color changes and shows an increase in chlorophyll density (assumed due to algae cell growth) in the systems, and the rate of CO2 uptake shows an increase for the darker, aged coatings (Figure 11 shows the data for the 1% algae sample).
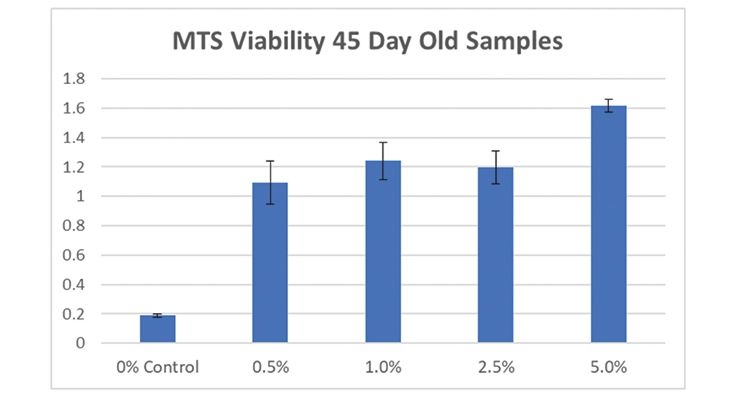
Figure 12 shows the color change for identical, freshly made samples compared to the 45-day old samples. MTS analysis was conducted on the 45-day old samples and confirmed that they still contained viable algae cells (Figure 13).
Isolation of Algae-Grown Cellulose from Gel-Coatings and Gravimetric Analysis
The genetically modified alga we used in this work was modified to express cellulose synthesis genes from Gluconacetobacter hansenii (ATCC 53582, formerly Gluconacetobacter xylinus), which naturally exudes a cellulose product. Figure 14 shows the Gluconacetobacter in a liquid culture with the cellulose pellicle (the lighter colored material floating on top of the liquid media) it produced over 14 days. While the genetically modified algae (overproducer) does not exude the cellulose to form a pellicle per se, the excess cellulose is secreted from the cell into an extracellular matrix. We have been able to (1) harvest the coatings from the bottles and then (2) extract the cellulose-enriched cells by simply reversing the ionic alginate crosslinks of the gel coating using common calcium chelators (Figure 15).
Vertically Scaled, Algae Gel-Coating Arrays
To tackle a problem of such an enormous scale as the anthropogenic CO2 released each year (approximately 50 gigatons), the solution must also be able to scale to significant proportions. This is where coatings truly shine – the ability to apply the carbon capture coating in multiple iterations of vertical structures (in our proof-of-concept model using stacked PET containers) allows significant capture of CO2 while using a smaller horizontal footprint. Considering our initial proof-of-concept system, we can easily scale our coated PET container array from 3 feet tall sleeves to 10 feet tall (Figure 16). For this very small-scale system that change would create a 3x multiplier in CO2 uptake without using any additional horizontal area footprint.* As surface area/volume is further maximized, additional efficiency in carbon capture can be realized. Additional benefits to this algae coating system include the fact that the thin coatings on the surfaces are much lower weight than equivalent liquid cultures (allowing taller vertical structures), and the water content of the coatings, while significant, are also greatly reduced from the amount of water used to create liquid algae cultures. The end use of the captured CO2 is dependent on the intended application and is where the flexibility of coatings is again a significant advantage. As discussed in the previous section, the current gel-coating system can have the crosslinks reversed allowing the collection of the carbon-containing product synthesized by the algae, or the entire coating system and could be used to make other materials (such as pressed boards, concrete, bricks, etc.) to sequester the carbon for a significant amount of time.
Conclusions and Call-to-Action
There is an enormous amount of paintable man-made surface area in the world. If the coatings adhered to such surfaces are dynamically functional, these surfaces can become enormous machines with which to accomplish otherwise difficult to imagine tasks. We have been conducting extensive research and development on how to use bio-based molecules (such as peptides, enzymes, and isolated cell parts) to program coated surfaces to repeatedly carry out useful dynamic functionality since we began in 2001. We now extend this work to include long-term inclusion of living cells into coatings. While this goal to create coatings that help reverse climate change might seem an impossible task, it is the first step in just such a plan we are researching and report here.
We decided to formulate compositions mimicking the carbon capturing rates of marine algae by entraining whole cell algae capable of capturing, fixing, and sequestering carbon dioxide into cellulose in a thin, super-hydrated coating. We’ve reported promising initial rates of CO2 uptake by lab-scale versions of these coated surfaces, and these surfaces are easily iterated to create extremely large amounts of coated surface area to potentially offset the large amounts of excess carbon dioxide we currently generate. Our results indicate that this goal can be met, and that if the manufacturing and distribution capabilities of the coatings industry can be rapidly engaged, it is possible to offset very large portions, if not all, of the excess carbon presently emitted annually. The rates of capture will be increased with experimentation, and as we and others have stated, should rival that of green plants at close to a 20-fold increased rate over that we initially have observed. The squared surfaces area to cubic volume ratios will be dramatically increased with simple engineering. As critically, the types of resin systems and the particulars of formulations will undoubtedly yet again increase the greenhouse gas capturing capabilities of these systems.
It is the lattermost issue where we hope to quickly gather a coating industry-wide consortium. Such a consortium can leverage our initial proof-of-concept findings into more efficient polymer systems. That consortium will be able to quickly determine the maximal means to drive economic value over-and-above the enormous benefits of mitigating global warming, including inputs from those along the paint supply chain to end users/consumers. Clearly, no one company can do what a consortium can rapidly achieve. A “landing site” for those interested in forming such a consortium can be found at www.reactivesurfaces.com/carbon_capture_coatings.
Materials and Methods Growth of Algal Strains
For algae growth media, Blue-Green Medium 11 (“BG-11”) liquid and solid media were prepared as described in literature.17 The primary photosynthetic organism evaluated was the algae Synechococcus leopoliensis [“wild type” strain UTEX 2434; source UTEX Culture Collection of Algae, The University of Texas at Austin, TX] or a genetically engineered variety of this strain modified to produce additional cellulose [“overproducer,” Synechococcus leopoliensis strain UTCC 100 modified with the cellulose synthesis genes (acsABΔC) from Gluconacetobacter hansenii (formerly Gluconacetobacter xylinus), ATCC 53582]4. Additional cyanobacteria tested in this study include Synechocystis sp. (UTEX 2470) and Agmenellum quadruplicatum (UTEX 2268). The algae were cultured either in flasks, in which the BG-11 medium volume was 1/5 the total flask capacity (e.g., 10 mL medium per 50 mL flask, which were incubated with gentle shaking at 100 revolutions per minute (rpm), or in 1 L bottles with 1 L BG-11 medium, which were incubated with filtered house air bubbling through them. In either culture technique, a culture was grown until it reached an optical density (OD) measured at 540 nm (OD540) of about 1. All cultures were incubated at ambient temperature (about 20-23 ºC) under grow lights [about 70 µmol photons m-2 s-1 photosynthetic active radiation (“PAR”) for flasks and 15 PAR for the 1 L bottles during growth from one or more 9.6 Watt extendable 16 inch emitting diode (“LED grow light strips (Litever, Shenzhen, Guangdong, China).
Preparation of Algae Wet Cell Pellet
To prepare wet cell pellets, the algal cultures that had reached OD540 of about 1 were centrifuged at 3000x gravity (g) for 15 minutes. The supernatant was poured off, and the remaining cell pellet was resuspended in the remaining liquid BG-11 medium for a total volume of approximately 1/100 of the original volume to make the wet cell pellet.
Preparation of Algae and Algae/Latex Coated Disks
Algal cells (Synechococcus leopoliensis UTEX 2434; Synechocystis sp. UTEX 2470; Agmenellum quadruplicatum UTEX 2268) were concentrated into wet cell pellets. One hundred microliters of the wet cell pellet was mixed with either 100 µL of BG-11 liquid medium or 100 µL of acrylic latex resin (Sherwin-Williams Company, Cleveland, Ohio) adjusted to pH 7. Algal medium or acrylic latex resin control samples were also prepared. The cell suspensions were pipetted onto 0.8 µm nylon filters (Millipore Sigma, Burlington, MA) about 10 cm2 in area. The filters were allowed to dry about 5-10 minutes, then placed onto the surface of BG-11 agar. The agar was cut around the filter so that the filter was sitting on an agar “puck.” The filter and agar puck were transferred to a 250 mL clear plastic chamber, and the CO2 concentration was measured using a Vernier Go Direct CO2 sensor (Vernier Software & Technology). The sensor recorded the CO2 concentration in ppm every 5 minutes for about an hour, which was plotted as ppm vs. time in minutes to get the rate of CO2 capture over time from the slope of the line. The CO2 capture of the no alga control latex resin was later subtracted from the CO2 capture of the alga/acrylic resin to determine the alga latex CO2 capture. This CO2 capture rate was used to calculate the CO2 capture rate as mmol CO2 hr-1 m-2.
Coating PET containers
BG-11 media (42 mL) was poured into a plastic cup and the desired amount (generally between 300 μL and 3.0 mL) of wet cell pellet of Synechococcus leopoliensis (UTEX 2434, wild type) or the algae overproducer was added and mixed. Lifemold alginate powder (6 g) was added to the plastic cup and stirred by hand with a wooden tongue depressor until mostly smooth. Pre-hydrated xanthan gum (12 g of 2 wt% mixture in BG-11 media) was added to the container and mixed until a smooth texture was achieved.
The pot-life of the gelation reaction is dependent on several factors (water temperature, ion content, water ratio, etc.), but generally the alginate converts from a liquid mixture to a gel within about 5 to 8 minutes after mixing with water. Then the mixture was poured into a PET container and the container was rotated continuously for several minutes until most or all the inside surface was coated by the mixture and the coating solidified.
CO2 Uptake Measurements
Carbon dioxide captured by coatings was measured using a Vernier® Go Direct CO2 sensor (Vernier Software & Technology, Beaverton, OR). For monitoring coated PET containers, they were placed in a plastic sleeve with end caps to seal the interior space of the plastic sleeve (sleeve and caps both available from Cleartec Packaging). One end cap was modified to fit the CO2 the sensor. The CO2 capture monitoring was generally conducted while the carbon dioxide capture devices were illuminated at 250 PAR by LED grow lights. The CO2 concentration in ppm was measured over a defined period of time using Vernier® Graphical Analysis software (Vernier Software & Technology). The ppm CO2 concentration was plotted against the time data to obtain a linear relationship, and the slope was measured as the capture of CO2 in ppm per unit time. This value was converted to a rate of mmol CO2 hr-1 m-2, with a positive rate occurring when carbon dioxide was captured. The rate in mmol CO2 hr-1 m-2 was based on the total volume of the device (e.g., coated container, coated PET container) in which measurement occurred as well as the total area in m2 that the carbon dioxide capture coating covered. The samples all showed slightly different beginning CO2 concentrations when monitored; therefore, when comparing samples measured at different times, the data was normalized to a 400 ppm CO2 starting concentration.
MTS Viability Assay of Algae Coatings
CellTiter 96® Aqueous One Solution (which contains the tetrazolium compound [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium; “MTS”] Promega Madison, WI) was used to assay the viability of the cells in the various formulations by coating the bottoms of the wells in a 96-well microplate with 0.05 g of coating for both control (no algae added) and polymer/algae coatings. To begin the assay, 100 µL of BG-11 liquid growth medium was added followed by 20 µL of the MTS solution to each well, and the plate incubated under normal indoor lighting at 25 °C with rocking in the MTS solution for 1 hour. After the incubation period, the contents were transferred to microcentrifuge tubes, centrifuged at 13,000 rpm for 10 minutes, and 60-80 µL of supernatant was transferred to new wells in a 96-well microplate. The absorbance at 492 nm was measured using a Multiskan Ascent microplate reader (Thermo LabSystems Inc. Beverly, MA). The change in absorbance is indicative of a change in cell proliferation with an increase in absorbance value resulting from increased cell metabolism.
Extraction of Cellulose from Algae Coatings
The genetically engineered overproducer was created to produce more significant amounts of cellulose than the wild-type algae (UTEX 2434).14 The coating used for these experiments also contains cellulose, but the cellulose included in the coating was accounted for by using coating-only controls or controls with the wild type algae. Because the alginate-based coatings crosslink using a multivalent ion (calcium in our formulation), chelators can be used to remove that multivalent ion from the gel and essentially return the gel to a liquid. After inhibiting the calcium crosslinkers, all the components of the coating formulation are soluble in water except the cellulose. We used two common calcium chelators to isolate the cellulose for our samples: 0.3 M ethylenediaminetetraacetic acid (EDTA) in deionized water (DI water) and 0.5 M sodium citrate in DI water. For a typical extraction, 5 g of gelled coating was added to a stirring mixture of 30 mL EDTA solution and 15 mL sodium citrate solution. Once the gel appeared dissolved (10-15 minutes) and a white-colored solid remained in the stirring solution, the entire contents of the extraction mixture was transferred to a centrifuge tube and centrifuged at 5,000 rpm for 15 minutes. The supernatant was poured from the centrifuge tube and the white powder was either transferred to a tared dish to dry or was left in the centrifuge tube to dry.
Acknowledgements
We would like to thank Lydia Howard, Dylan Reed, Aayushma Kunwar, and Nelson Conley our student interns from William Carey University, Hattiesburg, MS for their assistance with this project.
References
1. IPCC, 2018: Summary for Policymakers. In: Global Warming of 1.5°C. An IPCC Special Report on the impacts of global warming of 1.5°C above pre-industrial levels and related global greenhouse gas emission pathways, in the context of strengthening the global response to the threat of climate change, sustainable development, and efforts to eradicate poverty [Masson-Delmotte, V., P. Zhai, H.-O. Pörtner, D. Roberts, J. Skea, P.R. Shukla, A. Pirani, W. Moufouma-Okia, C. Péan, R. Pidcock, S. Connors, J.B.R. Matthews, Y. Chen, X. Zhou, M.I. Gomis, E. Lonnoy, Maycock, M. Tignor, and T. Waterfield (eds.)]. World Meteorological Organization, Geneva, Switzerland, 32 pp.
2. Santer, B. D.; Bonfils, C. J.; Fu, Q.; Fyfe, J. C.; Hegerl, G. C.; Mears, C.; Painter, J. F.; Po-Chedley, S.; Wentz, F. J.; Zelinka, M. D., Celebrating the anniversary of three key events in climate change science. Nature Climate Change 2019, 9 (3), 180.
3. Arenas, F.; Vas-Pinto, F., Marine algae as carbon sinks and allies to combat global warming. Marine algae: biodiversity, taxonomy, environmental assessment and biotechnology. CRC Press, Boca Raton 2014, 178-193.
4. Haoyang, C., Algae-Based Carbon Sequestration. IOP Conference Series: Earth and Environmental Science 2018, 120, 012011.
5. Eggleton, T. (2012) “A Short Introduction to Climate Change,” Cambridge University Press, p. 53; “Up-to-date weekly average CO2 at Mauna Loa” Earth System Research Laboratory Global Monitoring Division, Mauna Loa, Hawaii, Retrieved 03.28.19
6. Bridging the Emissions Gap: A UNEP Synthesis Report, Nairobi, Kenya: United Nations Environment Programme (UNEP), November 2011
7. Minx, J.C. et al., Environmental Research Letters 13(6):063001
8. Johnson, K.; Martin, D.; Zhang, X.; DeYoung, C.; Stolberg, A. Carbon dioxide removal options: a literature review identifying carbon removal potentials and costs. 2017.
9. Fuss, S.; Lamb, W. F.; Callaghan, M. W.; Hilaire, J.; Creutzig, F.; Amann, T.; Beringer, T.; de Oliveira Garcia, W.; Hartmann, J.; Khanna, T., Negative emissions—Part 2: Costs, potentials and side effects. Environmental Research Letters 2018, 13 (6), 063002.
10. Hawken, P., Drawdown: The most comprehensive plan ever proposed to reverse global warming. Penguin: 2017.
11. Smith, P.; Davis, S. J.; Creutzig, F.; Fuss, S.; Minx, J.; Gabrielle, B.; Kato, E.; Jackson, R. B.; Cowie, A.; Kriegler, E., Biophysical and economic limits to negative CO 2 emissions. Nature Climate Change 2016, 6 (1), 42.
12. Boysen, L. R.; Lucht, W.; Gerten, D.; Heck, V.; Lenton, T. M.; Schellnhuber, H. J., The limits to global-warming mitigation by terrestrial carbon removal. Earth’s Future 2017, 5 (5), 463-474.
13. McDaniel, S. and Blanton, M. “Functional coatings bring extended space missions closer.” Asia Pacific Coatings Journal Aug/Sept 2012, 25(4).
14. Nobles Jr, David R. and Brown Jr, R. M. “Transgenic expression of Gluconacetobacter xylinus strain ATCC 53582 cellulose synthase genes in the cyanobacterium Synechococcus leopoliensis strain UTCC 100” Cellulose 2008, 15:691–701. DOI 10.1007/s10570-008-9217-5
15. Bernal, O. I.; Mooney, C. B.; Flickinger, M. C., Specific photosynthetic rate enhancement by cyanobacteria coated onto paper enables engineering of highly reactive cellular biocomposite “leaves”. Biotechnology and bioengineering 2014, 111 (10), 1993-2008.
16. Lichen Biology. 2 ed.; Cambridge University Press: Cambridge, 2008.
17. Bustos, S.A. and Golden, S.S. Mol Gen Genet. 232:221–230, 1992.
*The model used in the proof-of-concept testing was not maximized in any way for rates of capture or surface to volume ratios. However, it may be instructive to use it to understand the power of coatings applied across large amounts of surface area to achieve impressive results. If one uses a cubic meter device occupying a square meter of horizontal ground space as the unitary carbon capture coating device, then one such device using the bottle-and-sleeve construct described here offers about a 50:1 vertical surface area to horizontal surface area advantage (when maximized this ratio will easily improve by at least a factor of 10). Operating such a device at a rate of capture (5 mmol CO2 hr-1 m-2) on a 12-hour per day basis for a year (4380 hours of capture per year) produces about 50 kg of a captured carbon per year. Thus, even at this modest rate, simple greenhouse-type structures sited upon non-arable land (e.g., that measure only 10 meters in length by 10 meters in width by 10 meters in height) would capture, fix, and sequester about 50 metric tons of CO2 per year.
Coatings capable of adhering to surfaces routinely exposed to light can exploit a biomimetic system similar to surfaces covered with photosynthetic lichen in order to capture, fix, and sequester carbon dioxide. When applied over sufficiently large amounts of surface area, such coatings are capable of rivaling natural carbon sinks in their ability to remove excess atmospheric carbon. The coatings industry stands in a unique position to cooperatively and profitably address climate change caused by excess carbon dioxide and to do so in a timely manner geared to preventing the most damaging of predicted environmental harm. By capturing large quantities of such greenhouse gases and sequestering the captured carbon into carbohydrates such as cellulose, these paints are capable of manufacturing useful byproducts, including some of the raw materials from which they themselves are formulated. This is the first technical description of these types of coatings.
Introduction
It is well known that carbon dioxide is a by-product of both naturally occurring and man-made activities. Examples of such naturally occurring activities include: animal respiration, decomposition of formerly living organisms, weathering of carbonate rocks, volcanic eruptions, and plant (e.g., forest) fire; examples of man-made activities (anthropogenic) include: fossil fuel use, intentional burning of biomass (e.g., wood stoves, intentional forest fires), and cement production. However, the current, drastic increase in carbon dioxide in the Earth’s atmosphere is being driven by release of additional carbon dioxide by mostly terrestrial, man-made activities, particularly in cities and industrialized regions. 1,2
Photosynthesis from land-based plants (including lichen and other cryptogamic species) and from ocean-bound algae (including cyanobacteria which are photosynthetic prokaryotes) is the primary, naturally occurring process for removing carbon dioxide from the Earth’s atmosphere. This naturally occurring photosynthetic conversion of carbon dioxide (CO2) requires water (which can be in the form of environmental moisture) and sunlight to convert available carbon dioxide in the atmosphere to oxygen (O2) and carbohydrates (e.g., saccharides). The ocean-bound algae are in the form of a thin layer floating at/near the surface of ocean water, known as the photic zone, and the vast size of the earth’s oceans provides a substantial surface area by which these ocean-bound algae can perform the majority (70 to 80%) of photosynthetic capture of atmospheric carbon dioxide and release of oxygen into the atmosphere. 3,4
However, because of the sheer magnitude of carbon-dioxide resulting from man-made activities, naturally occurring photosynthetic conversion of carbon dioxide has for many decades been unable to mitigate the amount of carbon-dioxide produced by man-made activities. As a result, excess amounts of carbon dioxide have built up in the atmosphere. It is estimated that the amount of carbon dioxide in the Earth’s atmosphere has increased from 280 parts per million (ppm) in the 1700s to 411 ppm as of March 2019.5 To make matters worse, approximately 50 gigatons of additional carbon dioxide equivalents (e.g., greenhouse gases such as carbon dioxide, methane, nitrous oxide, hydrofluorocarbons, perfluorocarbons, sulfur hexafluoride) are released into the Earth’s atmosphere yearly, primarily from fossil fuel usage.6 It is well known that carbon dioxide represents about 80% of “greenhouse gas,” with methane, nitrous oxide, and fluorinated gases representing the balance. It is also well known that these greenhouse gases are the primary contributing factor to global warming and the associated climate change around the globe (i.e., severe storms, warming of the oceans, melting of glaciers, rising sea levels). The current level of greenhouse gases and their projected rate of increase have led experts in the field of climatology to the conclusion that, if left unchecked, further increases in greenhouse gases will result in irreversible climatic changes and catastrophic effects of climate change on people, property, and the ecosystem.1,2
To combat the current level of greenhouse gases and their projected rate of increase, various forms of negative emission technologies are being developed. A negative emission technology removes (i.e., captures) carbon dioxide equivalents from the atmosphere where they may be sequestered or stored for long periods of time (i.e., years, decades, centuries or longer).7
Negative emission technologies can include enhanced carbon sinks that provide for long-term storage of the removed carbon dioxide (and/or other component(s) of greenhouse gas). Examples of enhanced carbon sinks include storage of captured carbon dioxide underneath the surface of the earth, conversion of captured carbon to useful solid or liquid materials such as plastics, carbon fibers, biofuel, or carbon-based chemicals.8,9 Some other negative emission technologies include: geological carbon sequestration, direct carbon dioxide capture from the atmosphere, bioenergy carbon capture and storage, coastal blue carbon capture, terrestrial carbon sequestration, and carbon mineralization of carbon dioxide.10
In much the same way that the magnitude of photosynthetic conversion of atmospheric carbon dioxide by ocean-bound algae is limited by the surface area of the ocean, known negative emissions technologies are limited in their efficiency and effectiveness for mitigating carbon dioxide by their available footprint (terrestrial or otherwise). Surface areas occupied by facilities housing these negative emissions technologies must do so in a manner that is practically feasible from societal and financial perspectives.11,12 Therefore, a negative emissions technological solution that simulates the unequalled efficiency and effectiveness of ocean-bound algae, but in a manner that provides for markedly greater surface area-to-volume practicalities, would be advantageous, desirable, and useful.
The coatings industry is a world-wide industry servicing paintable-surface-area measured in the billions of square meters, much if not most of which coated area is vertically oriented or otherwise incorporated into vertical structures such as buildings, glass windows, plastic components, etc. When coupled with myriad other types of articles of man-made manufacturing, the actual surface area coated yearly is staggering. Even more enormous is the true surface area of a porous coating itself, which cannot be seen by eye.13 To mimic this almost unlimited amount of vertical surface area to test the ability of carbon capture coatings to drawdown sizeable portions of anthropogenic carbon dioxide, an economical model system comprising translucent plastic sleeves containing translucent PET containers with easily measurable interior paintable surface area was constructed. While this model does not entirely mimic all surfaces amenable to carbon capture coating (CCC) coverage, it allows the construction of massively iterated vertical surface (MIVS) devices (CCC-MIVS) that may be useful to initiate a global-scale coating effort that can immediately be placed into service drawing down large amounts of CO2.
Experimentsm and Results
From the Ocean to a Flask
Culturing algae has long been performed with great success, and our collaboration with the Culture Collection of Algae at the University of Texas at Austin (UTEX; one of the most extensive collections of algae in the world) has provided us with access to thousands of potential algae candidates. Here we report our work with a particular algal (cyanobacterial) strain, Synechococcus leopoliensis (UTEX strain 2434, “wild type”). These algae have also been genetically engineered to produce additional cellulose (NS::abΔc7S, “overproducer”)14, and we examined both the wild type and the engineered cellulose overproducer in this work. Figure 1 shows both cultures being grown in glass jars under LED grow lights in our laboratory.
Out of the Flask and Almost a “Coating”
Bernal et al.15 reported the use of algae mixed 1:1 with a latex resin and coated onto paper in an effort to make a “synthetic cellular biocomposite leaf.” They reported CO2 uptake rates of up to 5.67 mmol CO2 hr-1 m-2 for cells kept hydrated by wicking an algae culture medium onto the coated filter paper in an artificially increased CO2 environment (20% CO2). These researchers stated their belief that similar systems should be capable of exceeding the rates of carbon capture seen in green plants such as Arabidopsis (18 mmol CO2 hr-1 m-2), and we agree. We chose to replicate their system using several test algae without the artificial CO2 environment to see if our rates of CO2 uptake would be similar. Figure 2 shows a typical filter paper sample containing either algae cells alone or a 1:1 wet mixture of algae cells and latex resin being measured for its CO2 uptake rate. The filters were kept moist by placing them on fresh agar pucks. Our CO2 uptake rates were very similar to those of Bernal et al. when tested for an hour of 250 PAR LED light exposure. These samples were followed with time, and the CO2 uptake rates are shown in Figure 3.
Once we had evidence of CO2 uptake for both the cells alone and the cells mixed with latex resin, we tested the viability of the algae cells mixed with BG-11 media, agar, or an acrylic latex resin as either dried films or in the wet state. We also tested the samples under different lighting conditions: under LED illumination or under ambient laboratory light. Each sample was run in triplicate, and controls for the BG-11, agar, and latex resin were included. Figure 4 shows the test plate after the MTS viability testing where a darker red/brown color indicates increased cell metabolism, which correlates with positive cell health and/or growth. Alcohol killed samples and heat killed samples were used as negative controls and showed no reaction in the test. Figure 5 shows the numerical comparison for the testing of the cells alone and with the polymer systems. In all cases, the wet films had higher performance (in some cases much higher performance) than the dry films. This led us to develop a highly-hydrated coating that did not require constant wicking of water or medium and that would better support the viability and CO2 uptake of the algae over long periods of time.
Super-Hydrated Algae Gel-Coatings
Based on the results in the MTS viability studies and our review of literature on lichens (that perform this function in nature),16 it was determined that high levels of hydration in the coating (80-100%) could lead to improved viability of the cells and increased photosynthesis. It was our goal to develop a coating based on natural materials from renewable resources, and we screened many water absorbing polymers. The system that best met our requirements was a blended system of sodium alginate (we chose a commercial, pre-formulated alginate) and xanthan gum. This system has many advantages: it can absorb many times its weight in water, is tolerant to moderate levels of ions and minerals (supplied in the media as nutrients for the algae), and gels into a pliable, semi-solid that can retain its shape on a vertically positioned surface – essentially a polymeric, hydrogel coating. The algae cells are dispersed in the media or water used to hydrate the coating, and upon the addition of the liquid, the alginate begins the crosslinking process with about 5-8 minutes of total working time before the material sets into a semi-solid state. Figure 6 shows the process of making the coating mixture and applying it to the PET containers.
Once the PET containers are coated with the algae-containing carbon capture coating, they can either be measured as single devices or placed into plastic sleeves and measured as a group of samples (Figure 7). The CO2 uptake rates are compared in Figure 8 showing the change in CO2 with time for a flat plastic dish containing the coating without algae and for one with algae. The rate over the one-hour measurement period for the control was slightly negative (CO2 released, no uptake), but the rate was 1.75 mmol CO2 per m2 of coating surface area (rate reported as mmol CO2 h-1 m-2) for the sample containing 2.5% algae.
Evidence of Algae Growth and Longevity in Gel-Coatings
Several formulations were tested on the PET substrates to understand CO2 capture rates of the coatings. As an example, we studied the algae level initially added to the coating to understand what level might provide optimal CO2 uptake and were pleased to see that the algae have an extended lifetime in this coating system. Pictured in Figure 9 is an array of sleeves of the coated PET containers, which show the samples on the day they were made (day 0), as well as on days 10 and 45 after the samples were made. From left to right in the photo, the samples contain increasing amounts of algae cells: 0.5%, 1.0%, 2.5%, and 5.0%.
As expected, the green color in the PET containers darkens as the amount of algae in the PET container increases. The CO2 uptake rates on day 0 (normalized to the same starting concentration in Figure 10), which were measured for each sleeve of PET containers (5 PET containers total), also shows an increase with the increasing amount of algae until the rate equalizes between the samples at 2.5% and 5% algae concentration. As the coated PET containers age, the color changes and shows an increase in chlorophyll density (assumed due to algae cell growth) in the systems, and the rate of CO2 uptake shows an increase for the darker, aged coatings (Figure 11 shows the data for the 1% algae sample).
Figure 12 shows the color change for identical, freshly made samples compared to the 45-day old samples. MTS analysis was conducted on the 45-day old samples and confirmed that they still contained viable algae cells (Figure 13).
Isolation of Algae-Grown Cellulose from Gel-Coatings and Gravimetric Analysis
The genetically modified alga we used in this work was modified to express cellulose synthesis genes from Gluconacetobacter hansenii (ATCC 53582, formerly Gluconacetobacter xylinus), which naturally exudes a cellulose product. Figure 14 shows the Gluconacetobacter in a liquid culture with the cellulose pellicle (the lighter colored material floating on top of the liquid media) it produced over 14 days. While the genetically modified algae (overproducer) does not exude the cellulose to form a pellicle per se, the excess cellulose is secreted from the cell into an extracellular matrix. We have been able to (1) harvest the coatings from the bottles and then (2) extract the cellulose-enriched cells by simply reversing the ionic alginate crosslinks of the gel coating using common calcium chelators (Figure 15).
Vertically Scaled, Algae Gel-Coating Arrays
To tackle a problem of such an enormous scale as the anthropogenic CO2 released each year (approximately 50 gigatons), the solution must also be able to scale to significant proportions. This is where coatings truly shine – the ability to apply the carbon capture coating in multiple iterations of vertical structures (in our proof-of-concept model using stacked PET containers) allows significant capture of CO2 while using a smaller horizontal footprint. Considering our initial proof-of-concept system, we can easily scale our coated PET container array from 3 feet tall sleeves to 10 feet tall (Figure 16). For this very small-scale system that change would create a 3x multiplier in CO2 uptake without using any additional horizontal area footprint.* As surface area/volume is further maximized, additional efficiency in carbon capture can be realized. Additional benefits to this algae coating system include the fact that the thin coatings on the surfaces are much lower weight than equivalent liquid cultures (allowing taller vertical structures), and the water content of the coatings, while significant, are also greatly reduced from the amount of water used to create liquid algae cultures. The end use of the captured CO2 is dependent on the intended application and is where the flexibility of coatings is again a significant advantage. As discussed in the previous section, the current gel-coating system can have the crosslinks reversed allowing the collection of the carbon-containing product synthesized by the algae, or the entire coating system and could be used to make other materials (such as pressed boards, concrete, bricks, etc.) to sequester the carbon for a significant amount of time.
Conclusions and Call-to-Action
There is an enormous amount of paintable man-made surface area in the world. If the coatings adhered to such surfaces are dynamically functional, these surfaces can become enormous machines with which to accomplish otherwise difficult to imagine tasks. We have been conducting extensive research and development on how to use bio-based molecules (such as peptides, enzymes, and isolated cell parts) to program coated surfaces to repeatedly carry out useful dynamic functionality since we began in 2001. We now extend this work to include long-term inclusion of living cells into coatings. While this goal to create coatings that help reverse climate change might seem an impossible task, it is the first step in just such a plan we are researching and report here.
We decided to formulate compositions mimicking the carbon capturing rates of marine algae by entraining whole cell algae capable of capturing, fixing, and sequestering carbon dioxide into cellulose in a thin, super-hydrated coating. We’ve reported promising initial rates of CO2 uptake by lab-scale versions of these coated surfaces, and these surfaces are easily iterated to create extremely large amounts of coated surface area to potentially offset the large amounts of excess carbon dioxide we currently generate. Our results indicate that this goal can be met, and that if the manufacturing and distribution capabilities of the coatings industry can be rapidly engaged, it is possible to offset very large portions, if not all, of the excess carbon presently emitted annually. The rates of capture will be increased with experimentation, and as we and others have stated, should rival that of green plants at close to a 20-fold increased rate over that we initially have observed. The squared surfaces area to cubic volume ratios will be dramatically increased with simple engineering. As critically, the types of resin systems and the particulars of formulations will undoubtedly yet again increase the greenhouse gas capturing capabilities of these systems.
It is the lattermost issue where we hope to quickly gather a coating industry-wide consortium. Such a consortium can leverage our initial proof-of-concept findings into more efficient polymer systems. That consortium will be able to quickly determine the maximal means to drive economic value over-and-above the enormous benefits of mitigating global warming, including inputs from those along the paint supply chain to end users/consumers. Clearly, no one company can do what a consortium can rapidly achieve. A “landing site” for those interested in forming such a consortium can be found at www.reactivesurfaces.com/carbon_capture_coatings.
Materials and Methods Growth of Algal Strains
For algae growth media, Blue-Green Medium 11 (“BG-11”) liquid and solid media were prepared as described in literature.17 The primary photosynthetic organism evaluated was the algae Synechococcus leopoliensis [“wild type” strain UTEX 2434; source UTEX Culture Collection of Algae, The University of Texas at Austin, TX] or a genetically engineered variety of this strain modified to produce additional cellulose [“overproducer,” Synechococcus leopoliensis strain UTCC 100 modified with the cellulose synthesis genes (acsABΔC) from Gluconacetobacter hansenii (formerly Gluconacetobacter xylinus), ATCC 53582]4. Additional cyanobacteria tested in this study include Synechocystis sp. (UTEX 2470) and Agmenellum quadruplicatum (UTEX 2268). The algae were cultured either in flasks, in which the BG-11 medium volume was 1/5 the total flask capacity (e.g., 10 mL medium per 50 mL flask, which were incubated with gentle shaking at 100 revolutions per minute (rpm), or in 1 L bottles with 1 L BG-11 medium, which were incubated with filtered house air bubbling through them. In either culture technique, a culture was grown until it reached an optical density (OD) measured at 540 nm (OD540) of about 1. All cultures were incubated at ambient temperature (about 20-23 ºC) under grow lights [about 70 µmol photons m-2 s-1 photosynthetic active radiation (“PAR”) for flasks and 15 PAR for the 1 L bottles during growth from one or more 9.6 Watt extendable 16 inch emitting diode (“LED grow light strips (Litever, Shenzhen, Guangdong, China).
Preparation of Algae Wet Cell Pellet
To prepare wet cell pellets, the algal cultures that had reached OD540 of about 1 were centrifuged at 3000x gravity (g) for 15 minutes. The supernatant was poured off, and the remaining cell pellet was resuspended in the remaining liquid BG-11 medium for a total volume of approximately 1/100 of the original volume to make the wet cell pellet.
Preparation of Algae and Algae/Latex Coated Disks
Algal cells (Synechococcus leopoliensis UTEX 2434; Synechocystis sp. UTEX 2470; Agmenellum quadruplicatum UTEX 2268) were concentrated into wet cell pellets. One hundred microliters of the wet cell pellet was mixed with either 100 µL of BG-11 liquid medium or 100 µL of acrylic latex resin (Sherwin-Williams Company, Cleveland, Ohio) adjusted to pH 7. Algal medium or acrylic latex resin control samples were also prepared. The cell suspensions were pipetted onto 0.8 µm nylon filters (Millipore Sigma, Burlington, MA) about 10 cm2 in area. The filters were allowed to dry about 5-10 minutes, then placed onto the surface of BG-11 agar. The agar was cut around the filter so that the filter was sitting on an agar “puck.” The filter and agar puck were transferred to a 250 mL clear plastic chamber, and the CO2 concentration was measured using a Vernier Go Direct CO2 sensor (Vernier Software & Technology). The sensor recorded the CO2 concentration in ppm every 5 minutes for about an hour, which was plotted as ppm vs. time in minutes to get the rate of CO2 capture over time from the slope of the line. The CO2 capture of the no alga control latex resin was later subtracted from the CO2 capture of the alga/acrylic resin to determine the alga latex CO2 capture. This CO2 capture rate was used to calculate the CO2 capture rate as mmol CO2 hr-1 m-2.
Coating PET containers
BG-11 media (42 mL) was poured into a plastic cup and the desired amount (generally between 300 μL and 3.0 mL) of wet cell pellet of Synechococcus leopoliensis (UTEX 2434, wild type) or the algae overproducer was added and mixed. Lifemold alginate powder (6 g) was added to the plastic cup and stirred by hand with a wooden tongue depressor until mostly smooth. Pre-hydrated xanthan gum (12 g of 2 wt% mixture in BG-11 media) was added to the container and mixed until a smooth texture was achieved.
The pot-life of the gelation reaction is dependent on several factors (water temperature, ion content, water ratio, etc.), but generally the alginate converts from a liquid mixture to a gel within about 5 to 8 minutes after mixing with water. Then the mixture was poured into a PET container and the container was rotated continuously for several minutes until most or all the inside surface was coated by the mixture and the coating solidified.
CO2 Uptake Measurements
Carbon dioxide captured by coatings was measured using a Vernier® Go Direct CO2 sensor (Vernier Software & Technology, Beaverton, OR). For monitoring coated PET containers, they were placed in a plastic sleeve with end caps to seal the interior space of the plastic sleeve (sleeve and caps both available from Cleartec Packaging). One end cap was modified to fit the CO2 the sensor. The CO2 capture monitoring was generally conducted while the carbon dioxide capture devices were illuminated at 250 PAR by LED grow lights. The CO2 concentration in ppm was measured over a defined period of time using Vernier® Graphical Analysis software (Vernier Software & Technology). The ppm CO2 concentration was plotted against the time data to obtain a linear relationship, and the slope was measured as the capture of CO2 in ppm per unit time. This value was converted to a rate of mmol CO2 hr-1 m-2, with a positive rate occurring when carbon dioxide was captured. The rate in mmol CO2 hr-1 m-2 was based on the total volume of the device (e.g., coated container, coated PET container) in which measurement occurred as well as the total area in m2 that the carbon dioxide capture coating covered. The samples all showed slightly different beginning CO2 concentrations when monitored; therefore, when comparing samples measured at different times, the data was normalized to a 400 ppm CO2 starting concentration.
MTS Viability Assay of Algae Coatings
CellTiter 96® Aqueous One Solution (which contains the tetrazolium compound [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium; “MTS”] Promega Madison, WI) was used to assay the viability of the cells in the various formulations by coating the bottoms of the wells in a 96-well microplate with 0.05 g of coating for both control (no algae added) and polymer/algae coatings. To begin the assay, 100 µL of BG-11 liquid growth medium was added followed by 20 µL of the MTS solution to each well, and the plate incubated under normal indoor lighting at 25 °C with rocking in the MTS solution for 1 hour. After the incubation period, the contents were transferred to microcentrifuge tubes, centrifuged at 13,000 rpm for 10 minutes, and 60-80 µL of supernatant was transferred to new wells in a 96-well microplate. The absorbance at 492 nm was measured using a Multiskan Ascent microplate reader (Thermo LabSystems Inc. Beverly, MA). The change in absorbance is indicative of a change in cell proliferation with an increase in absorbance value resulting from increased cell metabolism.
Extraction of Cellulose from Algae Coatings
The genetically engineered overproducer was created to produce more significant amounts of cellulose than the wild-type algae (UTEX 2434).14 The coating used for these experiments also contains cellulose, but the cellulose included in the coating was accounted for by using coating-only controls or controls with the wild type algae. Because the alginate-based coatings crosslink using a multivalent ion (calcium in our formulation), chelators can be used to remove that multivalent ion from the gel and essentially return the gel to a liquid. After inhibiting the calcium crosslinkers, all the components of the coating formulation are soluble in water except the cellulose. We used two common calcium chelators to isolate the cellulose for our samples: 0.3 M ethylenediaminetetraacetic acid (EDTA) in deionized water (DI water) and 0.5 M sodium citrate in DI water. For a typical extraction, 5 g of gelled coating was added to a stirring mixture of 30 mL EDTA solution and 15 mL sodium citrate solution. Once the gel appeared dissolved (10-15 minutes) and a white-colored solid remained in the stirring solution, the entire contents of the extraction mixture was transferred to a centrifuge tube and centrifuged at 5,000 rpm for 15 minutes. The supernatant was poured from the centrifuge tube and the white powder was either transferred to a tared dish to dry or was left in the centrifuge tube to dry.
Acknowledgements
We would like to thank Lydia Howard, Dylan Reed, Aayushma Kunwar, and Nelson Conley our student interns from William Carey University, Hattiesburg, MS for their assistance with this project.
References
1. IPCC, 2018: Summary for Policymakers. In: Global Warming of 1.5°C. An IPCC Special Report on the impacts of global warming of 1.5°C above pre-industrial levels and related global greenhouse gas emission pathways, in the context of strengthening the global response to the threat of climate change, sustainable development, and efforts to eradicate poverty [Masson-Delmotte, V., P. Zhai, H.-O. Pörtner, D. Roberts, J. Skea, P.R. Shukla, A. Pirani, W. Moufouma-Okia, C. Péan, R. Pidcock, S. Connors, J.B.R. Matthews, Y. Chen, X. Zhou, M.I. Gomis, E. Lonnoy, Maycock, M. Tignor, and T. Waterfield (eds.)]. World Meteorological Organization, Geneva, Switzerland, 32 pp.
2. Santer, B. D.; Bonfils, C. J.; Fu, Q.; Fyfe, J. C.; Hegerl, G. C.; Mears, C.; Painter, J. F.; Po-Chedley, S.; Wentz, F. J.; Zelinka, M. D., Celebrating the anniversary of three key events in climate change science. Nature Climate Change 2019, 9 (3), 180.
3. Arenas, F.; Vas-Pinto, F., Marine algae as carbon sinks and allies to combat global warming. Marine algae: biodiversity, taxonomy, environmental assessment and biotechnology. CRC Press, Boca Raton 2014, 178-193.
4. Haoyang, C., Algae-Based Carbon Sequestration. IOP Conference Series: Earth and Environmental Science 2018, 120, 012011.
5. Eggleton, T. (2012) “A Short Introduction to Climate Change,” Cambridge University Press, p. 53; “Up-to-date weekly average CO2 at Mauna Loa” Earth System Research Laboratory Global Monitoring Division, Mauna Loa, Hawaii, Retrieved 03.28.19
6. Bridging the Emissions Gap: A UNEP Synthesis Report, Nairobi, Kenya: United Nations Environment Programme (UNEP), November 2011
7. Minx, J.C. et al., Environmental Research Letters 13(6):063001
8. Johnson, K.; Martin, D.; Zhang, X.; DeYoung, C.; Stolberg, A. Carbon dioxide removal options: a literature review identifying carbon removal potentials and costs. 2017.
9. Fuss, S.; Lamb, W. F.; Callaghan, M. W.; Hilaire, J.; Creutzig, F.; Amann, T.; Beringer, T.; de Oliveira Garcia, W.; Hartmann, J.; Khanna, T., Negative emissions—Part 2: Costs, potentials and side effects. Environmental Research Letters 2018, 13 (6), 063002.
10. Hawken, P., Drawdown: The most comprehensive plan ever proposed to reverse global warming. Penguin: 2017.
11. Smith, P.; Davis, S. J.; Creutzig, F.; Fuss, S.; Minx, J.; Gabrielle, B.; Kato, E.; Jackson, R. B.; Cowie, A.; Kriegler, E., Biophysical and economic limits to negative CO 2 emissions. Nature Climate Change 2016, 6 (1), 42.
12. Boysen, L. R.; Lucht, W.; Gerten, D.; Heck, V.; Lenton, T. M.; Schellnhuber, H. J., The limits to global-warming mitigation by terrestrial carbon removal. Earth’s Future 2017, 5 (5), 463-474.
13. McDaniel, S. and Blanton, M. “Functional coatings bring extended space missions closer.” Asia Pacific Coatings Journal Aug/Sept 2012, 25(4).
14. Nobles Jr, David R. and Brown Jr, R. M. “Transgenic expression of Gluconacetobacter xylinus strain ATCC 53582 cellulose synthase genes in the cyanobacterium Synechococcus leopoliensis strain UTCC 100” Cellulose 2008, 15:691–701. DOI 10.1007/s10570-008-9217-5
15. Bernal, O. I.; Mooney, C. B.; Flickinger, M. C., Specific photosynthetic rate enhancement by cyanobacteria coated onto paper enables engineering of highly reactive cellular biocomposite “leaves”. Biotechnology and bioengineering 2014, 111 (10), 1993-2008.
16. Lichen Biology. 2 ed.; Cambridge University Press: Cambridge, 2008.
17. Bustos, S.A. and Golden, S.S. Mol Gen Genet. 232:221–230, 1992.
*The model used in the proof-of-concept testing was not maximized in any way for rates of capture or surface to volume ratios. However, it may be instructive to use it to understand the power of coatings applied across large amounts of surface area to achieve impressive results. If one uses a cubic meter device occupying a square meter of horizontal ground space as the unitary carbon capture coating device, then one such device using the bottle-and-sleeve construct described here offers about a 50:1 vertical surface area to horizontal surface area advantage (when maximized this ratio will easily improve by at least a factor of 10). Operating such a device at a rate of capture (5 mmol CO2 hr-1 m-2) on a 12-hour per day basis for a year (4380 hours of capture per year) produces about 50 kg of a captured carbon per year. Thus, even at this modest rate, simple greenhouse-type structures sited upon non-arable land (e.g., that measure only 10 meters in length by 10 meters in width by 10 meters in height) would capture, fix, and sequester about 50 metric tons of CO2 per year.