As the Coatings Industry strives to meet increasingly lower volatile organic compounds (VOC) targets, achieving good film formation in waterborne systems without sacrificing other coating properties has become difficult. While low-VOC coalescing agents have been developed, they often impact hardness and blocking resistance of the resultant coatings because they remain in the film. However, the discovery of unique multifunctional wetting agents that also aid film coalescence – “coalescing surfactants” – has made it possible to solve several formulating challenges by using a single efficient additive.
This paper will discuss the mechanism of latex film formation and describe how film coalescence is affected by solvents and traditional coalescing agents. Within this context, the chemical and physical properties of a few coalescing surfactants will be compared to those of common coalescing agents, and the mode of action of these remarkable additives will be explained. Furthermore, examples demonstrating how one can effectively formulate low-VOC industrial and decorative coatings using these coalescing surfactants will be described.
Dive Deeper: Sign Up To Be An Industry Insider
Introduction
Mechanism of Latex Film Formation and Achieving Film Coalescence
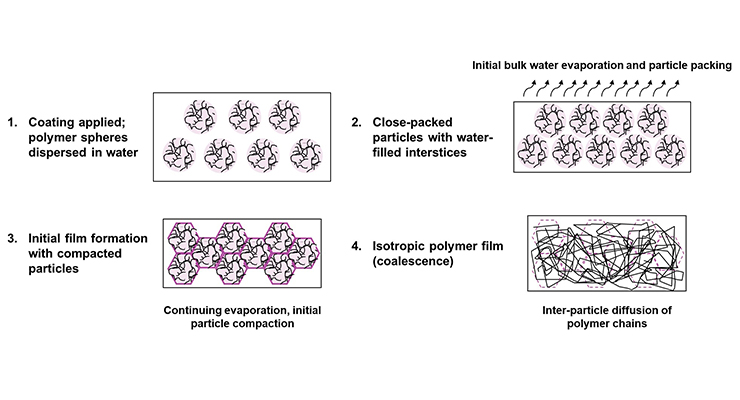
Polymer latex film formation has been shown to occur via the mechanism shown in Figure 1.1 When the coating is initially applied, it exists as an aqueous dispersion of discrete polymer spheres containing multiple polymer chains that begin to pack as the bulk of the water evaporates. Continuing evaporation causes the particles to closely pack and deform; however, film coalescence is only achieved when inter-particle diffusion of polymer chains occurs.
Polymer latexes can exist as both emulsions and dispersions and, while their mechanisms of film formation are similar, their physical characteristics are different. A polymer emulsion exists as stabilized liquid droplets in water and, as a result, has a glass transition temperature (Tg) below room temperature. Polymers with Tg values below application temperature can form fully closed films because the polymer molecules can diffuse between neighboring droplets. Even then, film formation and flow can benefit from small amounts of a coalescing agent – often also referred to as a coalescent. Polymer dispersions, on the other hand, have Tg values greater than room temperature and will require coalescing agents to film form unless curing at a temperature above the Tg of the polymer shell is possible. Higher coalescing agent levels are typically required for polymer dispersions than are needed for polymer emulsions. More complex polymer systems such as gradient and core-shell polymer dispersions are common as well, and these polymer particles can vary in properties from surface to center. Since coalescence typically occurs at the particle surface, the coalescent need only soften the shell to achieve good film formation.
The minimum film formation temperature (MFFT) of a coating is the lowest temperature at which a continuous film can form. If a coating is applied below its MFFT, it cannot coalesce and problems like “mud cracking” (Figure 2) will occur. A coalescing agent, like the tripropylene glycol n-butyl ether (TPnB) shown in Figure 2, decreases the Tg of the polymer and lowers the MFFT of the polymer dispersion. Coalescing agents with lower water solubilities have an increased ability to penetrate the resin from the aqueous phase because they prefer an organic phase. For example, dipropylene glycol dimethyl ether (DMM) and dipropylene glycol n-butyl ether (DPnB), which have water solubility limits of 35 wt.% and 4.5 wt.%, respectively, are not as efficient coalescing agents as 2,2,4-trimethyl-1,3-pentanediol monoisobutyrate (TMPiB), which has a water solubility limit of 0.12 wt.%.
Water soluble solvents like propylene glycol and butyl glycol can have a positive effect on coalescence even though they only penetrate the resin once the water has evaporated. Coalescence is heavily dependent on the remaining quantity of solvent and often higher solvent levels are required to effect coalescence compared to coalescing agents. The presence of water-soluble solvent can increase the solubility of a coalescing agent in the aqueous phase, hence decreasing the efficiency of the coalescing agent. Moreover, these solvents also contribute to VOCs, so their use must be carefully considered.2
No solvents or coalescing aidsCraterWith 2% TPnB, VOC = 200 g/L
Coalescing Surfactants and Their Role in Low-VOC Coatings
National and regional VOC and indoor air quality regulations are becoming increasingly restrictive, and, as a result, low-VOC coatings must minimize the use of volatile solvents and coalescing agents; however, this can result in poorer coating integrity and appearance if the coating is applied at or below its MFFT. Lower Tg polymer emulsions can be used without coalescing agents, but these polymer films tend to be softer and less durable.
Coalescing surfactants are wetting agents that are also capable of lowering a coating’s MFFT.3,4 As such, they reduce the surface tension of waterborne coatings at relatively low use levels (0.1 to 1.0 wt.%) and can enable the removal of some or all of the traditional coalescent, thus helping achieve target VOC levels while maintaining performance and reducing overall additives loadings. Ideally, the coalescing surfactant itself imparts little or no VOC to the formulation and has a minimal effect on the coating formulation or final performance properties.
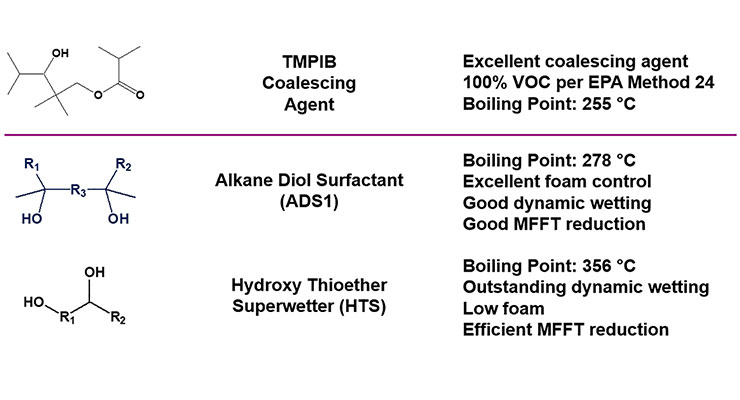
Representative structures of two coalescing surfactants are shown in Figure 3. In particular, the alkane diol surfactant ADS1 provides very good dynamic wetting and functions as an antifoam, while the hydroxy thioether superwetter HTS is an outstanding dynamic wetting agent that contributes no foam to the coating formulation.5 Both wetting agents additionally provide MFFT reduction to many different formulations as will be described herewith.
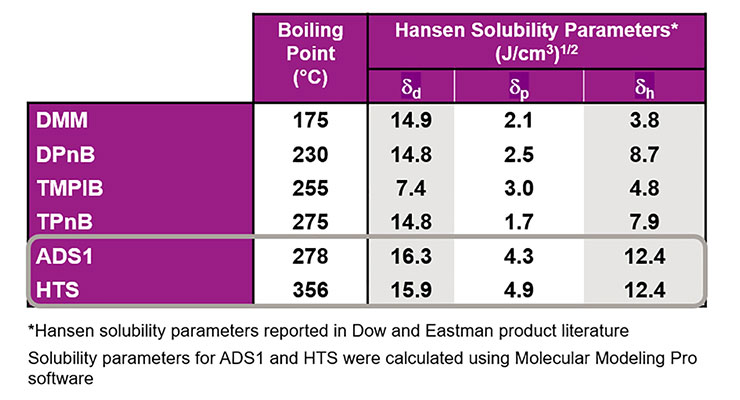
In recent years, Hansen solubility parameters of coalescing agents have been used to help identify the best coalescents for a particular polymer.6,7 As can be seen in Table 1, coalescing surfactants ADS1 and HTS have polar (p) and disperse (d) solubility parameters near those of traditional coalescing agents. Their somewhat larger hydrogen bonding parameters (h) correlate with the presence of the two hydroxyl groups present in ADS1 and HTS which are also responsible for their ability to perform as dynamic wetting agents.
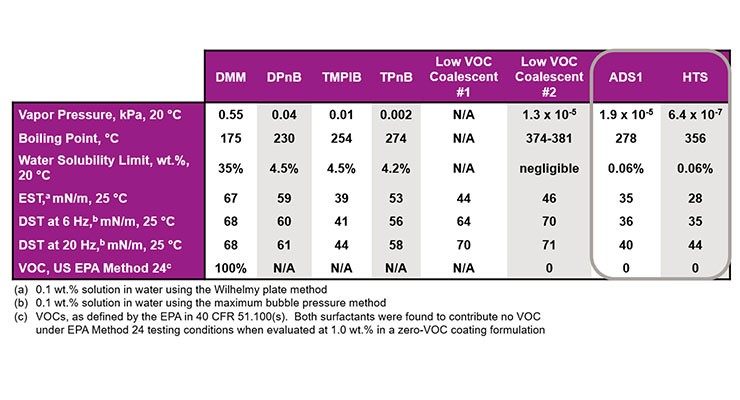
Table 2 compares some typical physical and chemical properties of the two coalescing surfactants with those of several standard coalescing agents and two low VOC coalescents. In addition to lowering both equilibrium (EST) and dynamic (DST) surface tension at 0.1 wt.% in water, both ADS1 and HTS have very low water solubility limits. Neither coalescing surfactant contributes to VOC as defined by EPA Method 24, and HTS has a high enough boiling point to meet even South Coast Air Quality Management District (SCAQMD) VOC regulations, as it contributes no VOC as confirmed using ASTM D6886 using methyl palmitate as a standard.
Results and Discussion
Experimental
Urethane-acrylic hybrid resins (Hybrid A, D, E and F) were provided by Evonik Corp., Acrylic B and Acrylic C were provided by Dow, the 100% self-crosslinking acrylic polymer emulsion was provided by Engineered Polymer Solutions & Color Corporation of America (EPS CCA), and the 100% acrylic latex was provided by Arkema. Coalescing solvents were procured from Dow or Eastman Chemical. Low LOV Coalescent #1 was provided by EPS CCA and Low VOC Coalescent #2 was provided by Eastman Chemical, while coalescing surfactants ADS1 and HTS were provided by Evonik Corp.
Equilibrium surface tension measurements were performed using a Krüss K100 tensiometer and the Wilhelmy plate method. Dynamic surface tension measurements were performed using a Krüss BP100 tensiometer and the maximum bubble pressure method. Surface energy was calculated using a Krüss DSA100 drop shape analyzer to measure the contact angles of water and diiodomethane on the substrate in question.
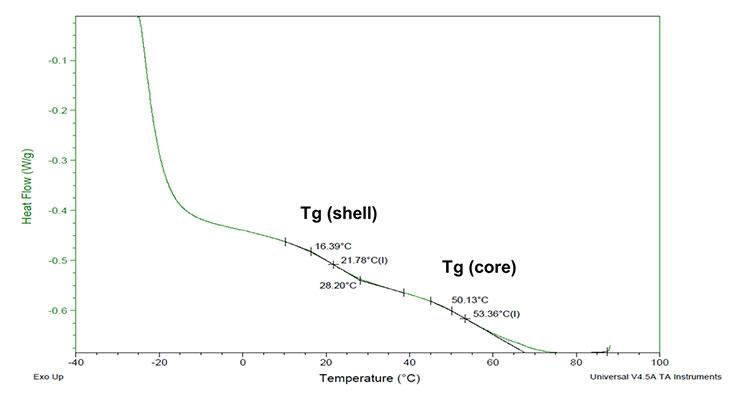
Glass transition temperatures were measured using a TA Instruments Q20 Dynamic Scanning Calorimeter (DSC). Liquid coating was added directly to the DSC pan, and the coating was taken from an initial temperature of -5 °C to an endpoint of 90°C using a 5 °C/min temperature ramp. Using this method, the Tg of both the shell and core of the polymer could be measured as shown in Figure 4.
Minimum film formation temperatures were measured using a Rhopoint Bar 90 MFFT unit that was purged with dry nitrogen. A 4-mil wet coating was applied to a 6 ½ x 17 inch Leneta black scrub test panel (Form P121-10N), and the drawdown was immediately taped to the equilibrated platen of the MFFT bar. The cover was closed, and the coating was cured until dry. Testing was performed using a range setting chosen so that the MFFT value fell in the center 1/3 of the platen. Range #2 (0 to 18 °C), Range #3 (5 to 23 °C) and Range #4 (15 to 33 °C) were used.
Study of the Effects of Coalescing Surfactants in Low-VOC Waterborne Clear Coating Formulations
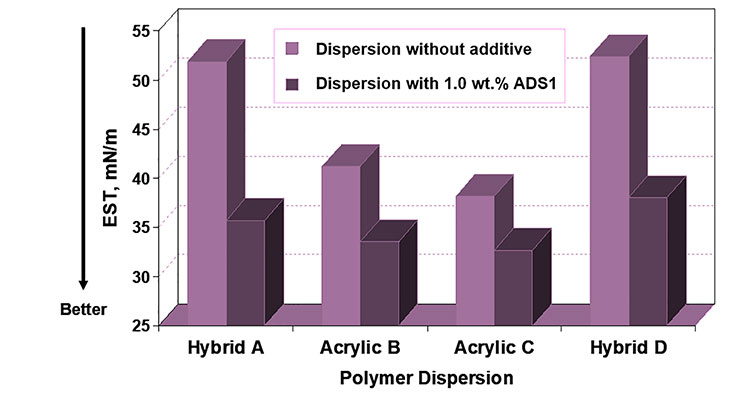
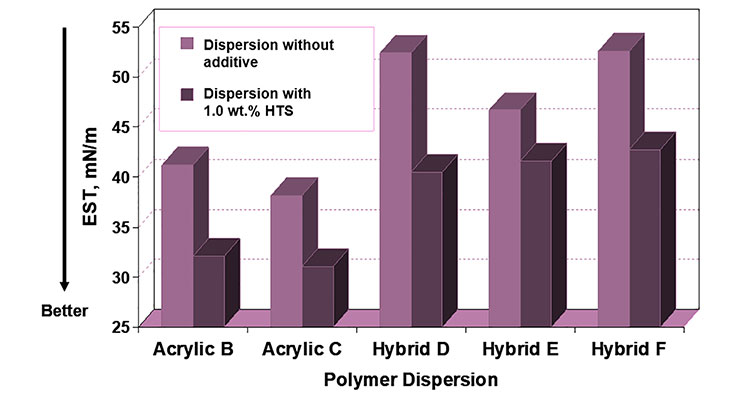
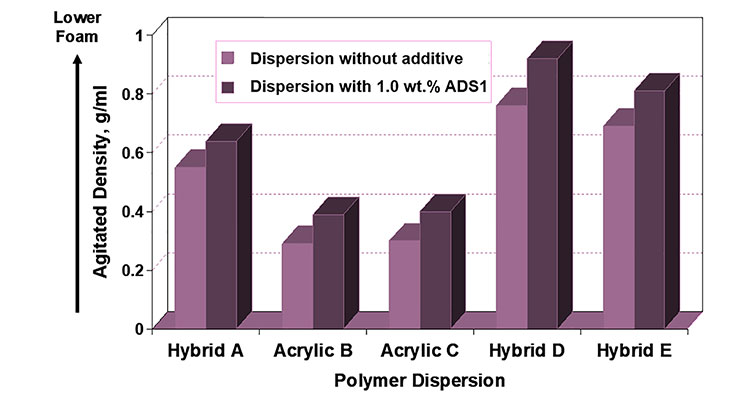
Initial evaluations of coalescing surfactants ADS1 and HTS focused on their ability to perform as wetting agents. As can be seen in Figures 6 and 7, both ADS1 and HTS can significantly lower the equilibrium surface tension of various aqueous polymer dispersions. Additionally, ADS1 acts as an antifoam as can be seen in Figure 8.
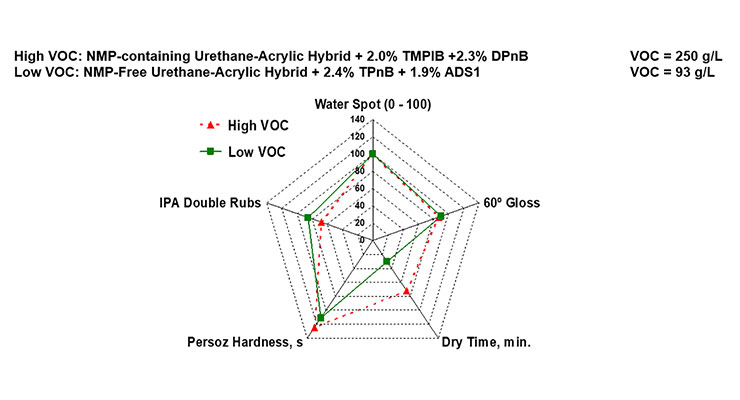
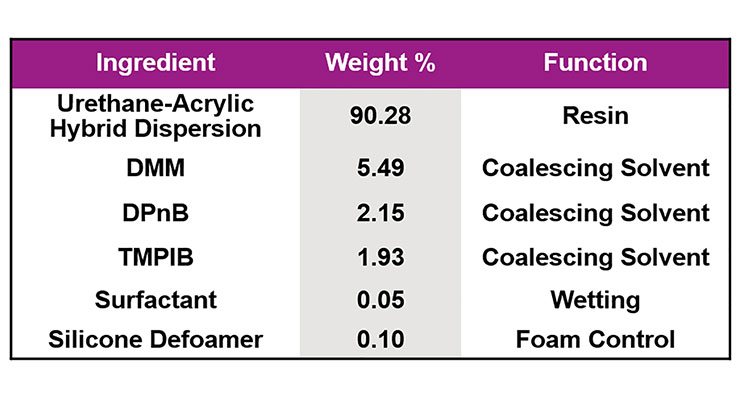
Clear coatings based on an NMP-containing urethane-acrylic hybrid resin and an NMP-free urethane-acrylic hybrid were then studied in order to determine if a low-VOC coating could be developed using the latter. As can be seen in Figure 9, using 1.9 wt.% of ADS1 as a dynamic wetting agent and antifoam enabled the removal of enough coalescing solvent that a <100 g/L coating that matched the physical properties of the high VOC alternative could be produced. While dry time of the low VOC coating was shorter, this was later improved through formulation optimization.
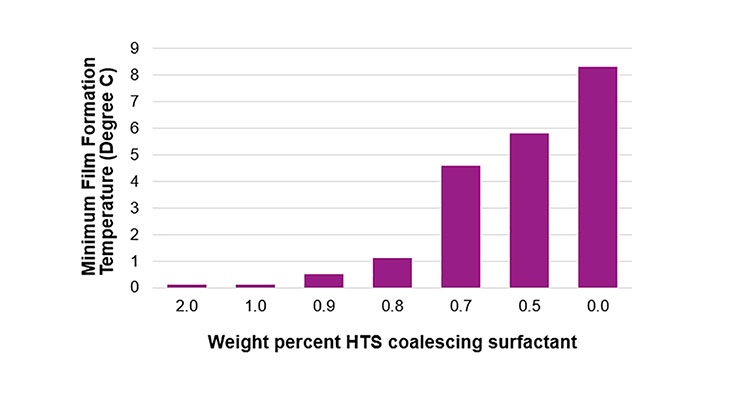
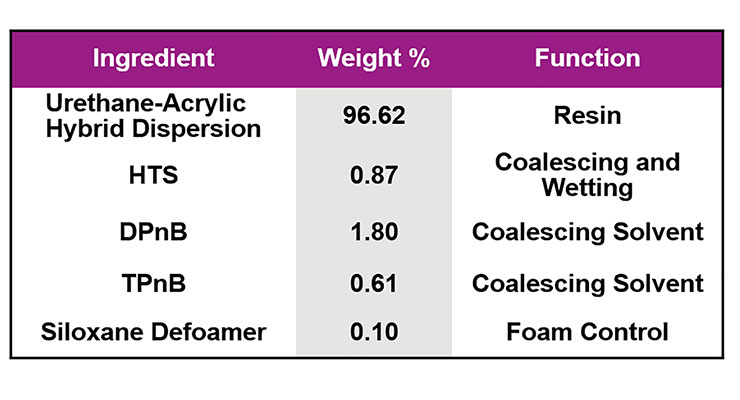
The superwetter HTS proved to be particularly efficient at lowering the MFFT in waterborne urethane-acrylic hybrid systems. In Figure 10, the effect of increasing levels of HTS on a low VOC clear coat can be seen. At typical surfactant use levels – less than 1 wt.% of the formulation – significant MFFT reduction is possible.
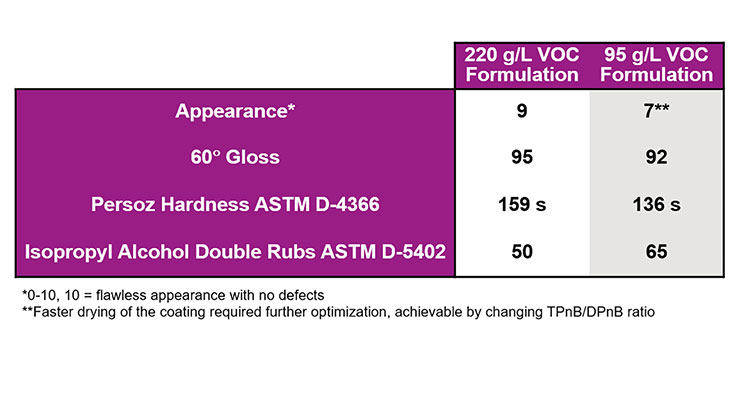
The two urethane-acrylic formulations in Table 3 and 4 were prepared and the resulting coatings’ properties were evaluated and are compared in Figure 11.
Study of the Coalescing Effect of HTS Coalescing Surfactant in Acrylic Interior/Exterior Gloss Paints
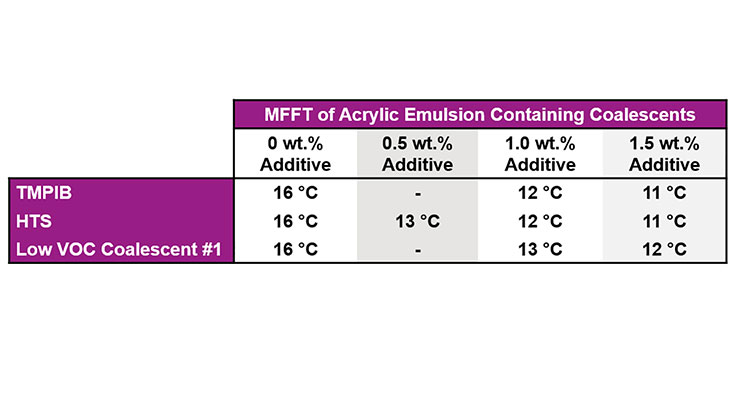
Since most US architectural coatings are now <50 g/L VOC, efforts next focused on studying the effects of the least volatile coalescing surfactant, HTS, in architectural paints. As can be seen in Tables 5 and 6, HTS, like TMPIB and Low VOC Coalescent #1 can lower the Tg of the polymer shell, and, hence, the MFFT of the acrylic emulsion. Since TMPIB is considered a VOC, it can only be used in small amounts in US architectural coatings and Low VOC Coalescent #1 provides MFFT reduction but is not multifunctional. Therefore, using HTS to provide dynamic wetting as well as coalescence can offer the formulator significant benefits.
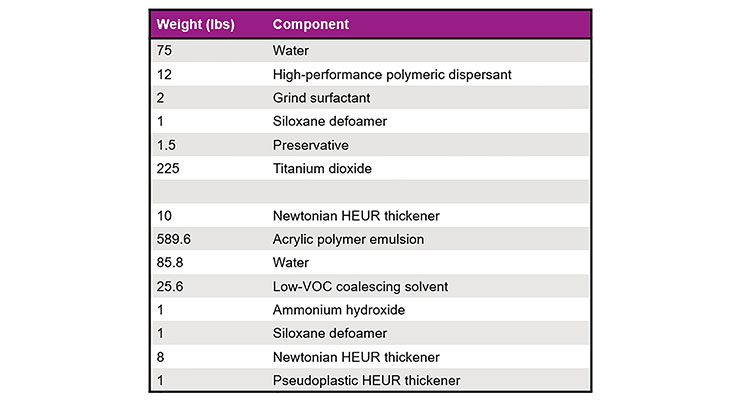
Table 7 shows a very low VOC (< 5 g/L) gloss paint formulation that was adapted from the acrylic emulsion supplier’s technical data sheet. It contains 2.5 wt.% Low VOC Coalescent #1 in the final formulation and, as prepared, it has an MFFT of 9 °C. Removing the grind surfactant and coalescing solvent from the formulation resulted in a base paint with an MFFT of 25 °C. However, post-addition of HTS not only provides the dry pigment wetting and deaeration needed from a grind surfactant but it also decreases the MFFT of the formulation as is shown in Figure 12. Optimizing the use level of HTS can ensure that its wetting and coalescing benefits are realized without adversely affecting blocking.
Table 8 shows an 18% PVC low-VOC gloss paint using a different acrylic latex which has a Tg of 29 °C and an MFFT of 20 °C. The latex supplier includes 2.9 wt.% of Low VOC Coalescent #2 in the starting point formulation upon which this formulation was based; however, it was omitted so that coalescing additives could be evaluated as post-additions.
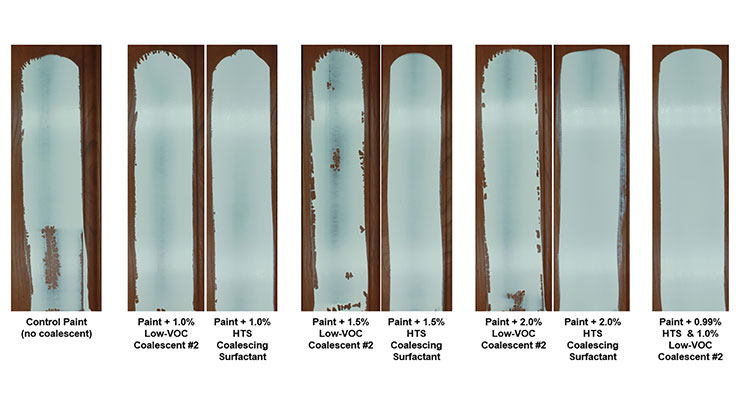
Addition of increasing amounts of either Low-VOC Coalescent #2 or HTS result in coatings with correspondingly lower MFFT values as can be seen in Figure 13. That said, Low VOC Coalescent #2 does not improve the wetting ability of coatings, while HTS is an efficient superwetter. The additional benefits of using HTS in this 100% acrylic gloss paint become apparent when the coatings are applied to hydrophobic substrates as can be seen in Figure 14. While excellent wetting can be achieved using 1.5 and 2.0 wt.% HTS as the sole coalescent, perfect wetting can also be achieved by using 1 wt.% HTS in combination with 1 wt.% of Low-VOC Coalescent #2. This combination demonstrates how coalescing surfactants can most effectively be used – adding enough of the coalescing surfactant to achieve the required wetting performance and then lowering the MFFT to the needed temperature through use of lower amounts of another low-VOC coalescent or a very small amount of a more volatile coalescing agent.
Conclusions
The multifunctionality of coalescing surfactants like HTS provides significant advantages in low-VOC coatings. HTS contributes no VOC and is a highly efficient dynamic wetting agent that enables rapid dry solids incorporation, enabling complete particle wetting and deaeration; its unique chemical structure allows it to provide surface tension reduction from grind to letdown to application. Because it is also an effective coalescent, using HTS as a wetting agent enables the removal of some, if not all, of the coalescing solvent or low-VOC coalescent. Additionally, the non-foam stabilizing, non-emulsifying, hydrophobic chemistry of HTS prevents it from causing foam, contributing to water sensitivity, or causing some of the other issues that can be seen with traditional surfactants.
The benefits of using coalescing surfactants can be maximized by adding them to the grind to achieve rapid dry pigment wetting and deaeration. This should enable replacement of some surfactant(s) used in milling and may enable use of less defoamer in the grind. The coalescing surfactant should be used in the amount needed to achieve dry pigment wetting – typically ≤ 1 wt.%; wetting benefits will carry through to final substrate wetting, minimizing the need for additional surfactants. Finally, the MFFT of the formulation should be checked and, if needed, a little traditional coalescent should be added to achieve the required MFFT.
Acknowledgments
We would like to thank Khalil Yacoub, Nancy Martin, Tony Hazim, Michael Pauley and Isabella Miserocchi for their technical assistance.
References
1. R.M. Rynders, C.R. Hegedus, A.G. Gilicinski, “Characterization of particle coalescence in waterborne coatings using atomic force microscopy,” Journal of Coatings Technology, 1995, 67 (845), 59-69.
2. W. Stout, “Reaching lower VOC targets with proper coalescence agent selection,” SpecialChem Webinar, 2011.
3. E.C. Galgoci, K. Yacoub, V.V. Shah, R. Reinartz, K.A. Yoxheimer, S.Y. Chan, “Multifunctional, Gemini-type coalescing surfactants enable formulation of lower VOC waterborne coatings,” JCT CoatingsTech, 2006, 3 (1), 34-42.
4. E.C. Galgoci, K. Yacoub, V.V. Shah and I.K. Meier, “Next generation coalescing surfactants for formulating low VOC coatings,” Presentation at the 2007 Western Coatings Symposium, 2007.
5. L. Herschke and I.K. Meier, “The next generation superwetter for waterborne coatings,” Parallel Session XI--Waterborne Systems, European Coatings Show, Nürnberg Congress, Nürnberg, Germany, 2007.
6. C.M. Hansen, “50 Years with solubility parameters – past and future,” Progress in Organic Coatings, 2004, 51, 77-84.
7. S. van Loon and B. Fricker, “Using HSP to select coalescents and improve film formation,”
https://coatings.specialchem.com/tech-library/article/using-hsp-to-select-coalescents-and-improve-film-formation 2020.
Technical Papers
Film Coalescence and Coalescing Surfactants:
Mechanism of Action and Practical Application of These Unique Multifunctional Wetting Agents in Low-VOC Coatings
Ingrid K. Meier, K. Michael Peck, J. Renae Bennett and Jonathan Sefko, Evonik Corporation01.18.22