Gary Shawhan, Chemark Consulting11.15.23
European regulations continue to play the leading role for the global coatings market in defining and setting standards for compliance. This article addresses several new initiatives that will have a significant impact on our industry. We have interviewed Ray Rex, of CRC-US, and Lars Dobertin, general manager of the CSB Compliance Group, Krefeld Germany to develop this information.
Rex: In December 2022, the European Parliament, the Council of the European Union and the European Commission presented a declaration on the EU’s legislative priorities for the next years. Implementing the “Green Deal” concepts through related legislative initiatives remains a top political priority. The “Green Deal’s” primary focus is on driving major revisions to EU REACH and CLP (European Classification, Labeling and Packaging) regulations.
The “Green Deal” also includes addressing other issues such as further chemical regulations related to microplastic pollution or sustainable product design. The fight against environmental crime represents another “Green Deal” focus. The Environmental Criminal Regulations will be reviewed to better address violations of REACH, BPR, and RoHS.
Although discussions have been progressing at a fast pace in these past two years, Europe remains far behind its self-anticipated schedule for the revisions to REACH. A draft that was expected initially by the end of 2022 has already been pushed back to the end of 2023. It is likely that “hands-on” regulatory text will not be published until after the European elections in mid-2024.
CW: Are there any key issues of critical importance to our industry?
Rex: Polymer registrations are a key element in this directive. Suppliers will be required to provide data on the toxicity of the polymers they manufacture and sell. The expectation is that about 50-60% of the polymers now sold will fall into a category of polymers NOT requiring registration (PRR-polymers requiring registration). These polymers will still need an initial notification to be submitted by each company, including supporting analytical data and a PRR assessment.
A second category will likely identify unique polymers. In these cases, the burden of data generation will rest with the individual manufacturer. This group would account for the remainder or an estimated 40-50%. As an indicator of the scope of the cost to address compliance, when these new regulations are finalized and implemented, it is estimated that there are approximately 200,000 polymers in use today within the EU.
Finally, once the dust settles, it is estimated that 10-20% of the polymers in use will require full registration under the new regulations.
CW: Can you offer any insight into how the polymers would be defined in these regulations?
Rex: The expectation is that polymers will be broken out into groups that represent a consensus of inputs from manufacturers, trade organization and other industry connected sources. These individual polymer groups will facilitate the use of consortiums to mitigate the individual costs for data generation
where possible.
As an example, there may be seven or eight categories of polymers listed in the regulations within which there will likely be multiple sub-categories. These categories will define the polymer information profiles and testing protocols required for registration by REACH.
CW: Is there a conceptual timeline for the REACH update?
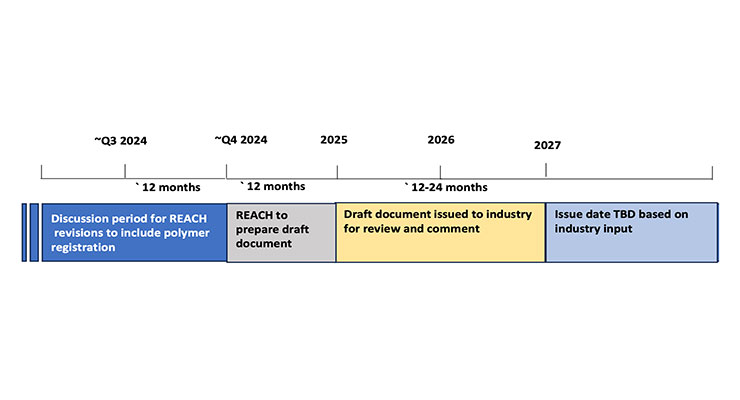
Rex: The process for moving forward with the planned updates to REACH is fluid and dependent on many factors including the time it will take to consider all industry inputs. The following graphic offers a conceptual timeline based on current information.
CW: Are there differences between REACH and Brexit on how they are planning to address the issues of polymer registration?
REX: UK authorities pushed back the chemical registration scheme effectively by three years. However, new substances to the UK must register before importing over 1MT to the UK. While the current registration scheme still closely follows that of Europe, very recent news indicates the UK and Brexit will shift away from a hazard data-driven-to-use and exposure-driven registration effort. While involved parties are highlighting potentially huge cost savings by this effort, it remains to be seen what the change will finally look like. More information will possibly be published in early 2024.
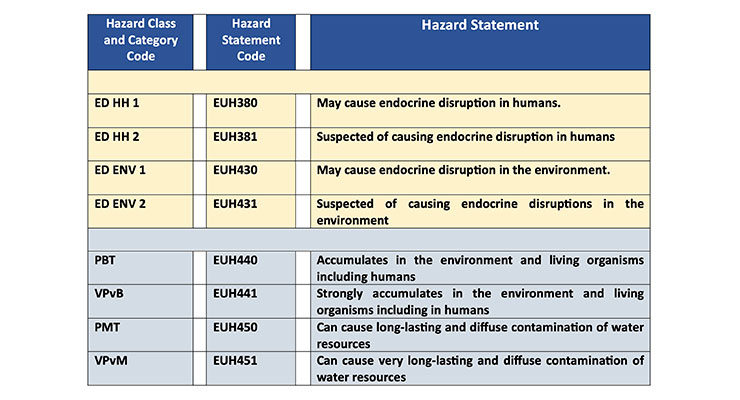
Rex: The new hazard classifications concern endocrine disruptors. These are substances that damage hormonal balance in humans or in the environment. These new classes also include persistent, very mobile disruptors that are highly mobile in soils. This can allow them the potential to enter drinking water. In addition, the new cases also include those substances that are toxic and bioaccumulate (Table 1).
CW: Can you elaborate on the objectives and the industry impact of the new labeling and packaging classifications?
Rex: A key objective for adding new categories of endocrine disruptors was to ensure that specific attention was placed of those materials that were very mobile and those that have issues with bioaccumulation. This includes their behavior in humans as well as the environment. This also highlights the severity that the EU is attributing to this specific hazard class, which already meets the SVHC (substance of very high concern) criteria.
Another aspect of these new hazard classifications is the expanded requirements for testing of smaller volumes of imported product than had been required in the past. Testing protocols will now be required for import quantities of 1-10 MT and 10-100 MT. This is in addition to the traditional 100-1000 MT, 1,000-10,000 MT and 10,000+ MT
volume categories.
The EU is currently still lacking data on ED substances (as strange as it may sound) since only registered high volume imported substances have been tested up till now. This is bound to change, possibly soon, with new ED test requirements are bound to be included in the 1-10MT and 10-100MT test regimes under REACH.
The addition of these lower annual import volume quantities is likely to have a significant effect on the economic viability of certain imported products that are consumed in small quantities.
CW: What is the ECHA timeline for implementation of these new Classifications?
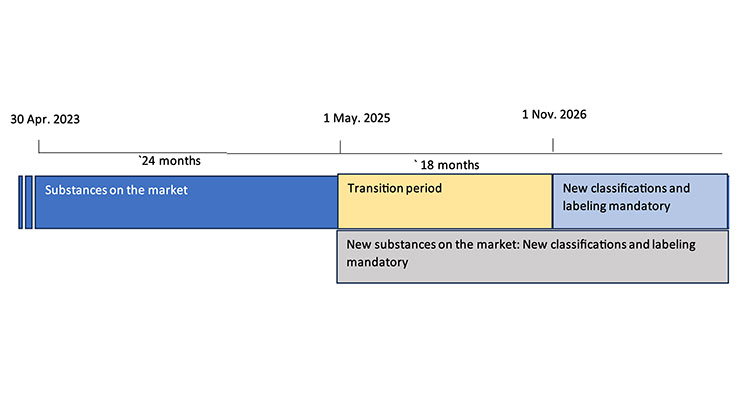
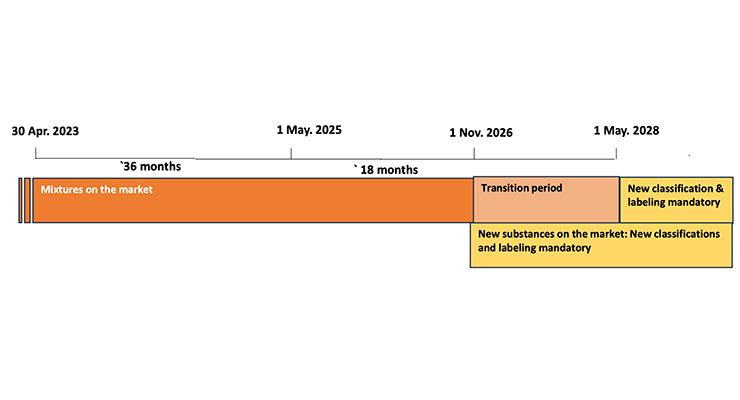
Rex: Figures 2 and 3 provide the ECHA (European Chemical Agency) timeline for reaching mandatory compliance with the revised regulations for the new hazard classifications and labeling require requirements.
Reach Regulation Revisions and Recent Changes
CW: How is REACH addressing sustainability?
Rex: Sustainability (within REACH) is one of the most far-reaching changes that is pending with the revision of the REACH Regulation. This effort is driven by public concerns regarding the potential adverse impacts of chemicals on the environment and human health. It is also elevated in importance by the political climate across the EU. The main objectives of an amendment to the REACH Regulation follows the 2020 “Chemicals Strategy for Sustainability” policy goals (CSS) (EC2020b).
CW: Can you elaborate on the current status of the sustainability initiative?
Rex: Consequently, the focus of this effort will be on measures for the targeted implementation of requirements for so-called “safe and sustainable by design chemicals.” It will also include the introduction of “non-toxic material cycles” and the establishment of an “essential use” concept. In addition, the most hazardous substances as well as endocrine disruptors and evaluation criteria for mixtures will be considered.
Studies commissioned in connection with the revision have largely been completed, whereby the evaluation of a reform of authorizations and restrictions as well as the support regarding the impact assessment are still open. After various delays, the draft of the intended amendments is expected in the course of this year.
The adoption of a corresponding proposal by the Commission is now expected for the last quarter of 2023 according to the Commission Work Programme for 2023 of 18.10.2022.
CW: What about biocides?
Rex: The European Biocidal Products Regulation (BPR) of September 2013 requires active biocide approval (Article 59 listing) followed by the authorization of the biocide product.
The process for approving new biocides has not changed since its inception in 2013 but new requirements for approving new biocides (e.g.. ED assessment) have been implemented in the meantime making it more complicated and costly.
The on-going active substance review program, which was targeted to end in 2024, will be prolonged as authorities are not able to assess the remaining “actives” within this timeframe. This is a “big deal” as they are now conducting the evaluations to complete the active chemicals permitted for use. Completion of this scheme will be pushed off
beyond 2024.
Once this list is set, its impact will be felt through restrictions on new biocide “actives,” which are to be approved first (the process currently taking many years) before marketing is allowed. The expense and time required to bring a new biocide to the market will be extremely high and likely prohibitive.
CW: What is the Candidate List of Substances for Authorization?
Rex: The REACH regulation maintains three separate lists with different requirements for the substances:
• The Candidate List (SVHC),
• Authorization (ANNEX XIV), and
• Restricted (Annex XVII)
The candidate list (SVHC) is the compilation of substances found to be carcinogenic, mutagenic or toxic to reproductive health through the “evaluation” phase of REACH. Any substance that is persistent, bio accumulative and toxic or is very persistent and very bio accumulative may also become an SVHC. SVHC’s are on the Candidate list because they have become candidates for Authorization, Annex XIV of the REACH regulation.
Note that the new CLP has new classifications for substances that are endocrine disruptors, persistent toxic, bio accumulative, or mobile. It is expected that the endocrine disruptor focus will place more substances on the list.
The Candidate list has been updated 29 times, the last being June 14, 2023, and currently contains 235 substances. See the link for a full listing of chemicals - Candidate List of substances of very high concern for Authorisation - ECHA (europa.eu). The REACH candidate list is the authentic version of chemicals under consideration.
REACH Activity Regarding Polymer Classifications And Regulations
CW: What is the “Green Deal”?Rex: In December 2022, the European Parliament, the Council of the European Union and the European Commission presented a declaration on the EU’s legislative priorities for the next years. Implementing the “Green Deal” concepts through related legislative initiatives remains a top political priority. The “Green Deal’s” primary focus is on driving major revisions to EU REACH and CLP (European Classification, Labeling and Packaging) regulations.
The “Green Deal” also includes addressing other issues such as further chemical regulations related to microplastic pollution or sustainable product design. The fight against environmental crime represents another “Green Deal” focus. The Environmental Criminal Regulations will be reviewed to better address violations of REACH, BPR, and RoHS.
Although discussions have been progressing at a fast pace in these past two years, Europe remains far behind its self-anticipated schedule for the revisions to REACH. A draft that was expected initially by the end of 2022 has already been pushed back to the end of 2023. It is likely that “hands-on” regulatory text will not be published until after the European elections in mid-2024.
CW: Are there any key issues of critical importance to our industry?
Rex: Polymer registrations are a key element in this directive. Suppliers will be required to provide data on the toxicity of the polymers they manufacture and sell. The expectation is that about 50-60% of the polymers now sold will fall into a category of polymers NOT requiring registration (PRR-polymers requiring registration). These polymers will still need an initial notification to be submitted by each company, including supporting analytical data and a PRR assessment.
A second category will likely identify unique polymers. In these cases, the burden of data generation will rest with the individual manufacturer. This group would account for the remainder or an estimated 40-50%. As an indicator of the scope of the cost to address compliance, when these new regulations are finalized and implemented, it is estimated that there are approximately 200,000 polymers in use today within the EU.
Finally, once the dust settles, it is estimated that 10-20% of the polymers in use will require full registration under the new regulations.
CW: Can you offer any insight into how the polymers would be defined in these regulations?
Rex: The expectation is that polymers will be broken out into groups that represent a consensus of inputs from manufacturers, trade organization and other industry connected sources. These individual polymer groups will facilitate the use of consortiums to mitigate the individual costs for data generation
where possible.
As an example, there may be seven or eight categories of polymers listed in the regulations within which there will likely be multiple sub-categories. These categories will define the polymer information profiles and testing protocols required for registration by REACH.
CW: Is there a conceptual timeline for the REACH update?
Rex: The process for moving forward with the planned updates to REACH is fluid and dependent on many factors including the time it will take to consider all industry inputs. The following graphic offers a conceptual timeline based on current information.
CW: Are there differences between REACH and Brexit on how they are planning to address the issues of polymer registration?
REX: UK authorities pushed back the chemical registration scheme effectively by three years. However, new substances to the UK must register before importing over 1MT to the UK. While the current registration scheme still closely follows that of Europe, very recent news indicates the UK and Brexit will shift away from a hazard data-driven-to-use and exposure-driven registration effort. While involved parties are highlighting potentially huge cost savings by this effort, it remains to be seen what the change will finally look like. More information will possibly be published in early 2024.
Expanded Hazard Classifications Under the Global Harmonization System for Hazard Communications
CW: What is the intent of the new hazard classifications for labeling and packaging (CLP) for chemicals entering the EU?Rex: The new hazard classifications concern endocrine disruptors. These are substances that damage hormonal balance in humans or in the environment. These new classes also include persistent, very mobile disruptors that are highly mobile in soils. This can allow them the potential to enter drinking water. In addition, the new cases also include those substances that are toxic and bioaccumulate (Table 1).
CW: Can you elaborate on the objectives and the industry impact of the new labeling and packaging classifications?
Rex: A key objective for adding new categories of endocrine disruptors was to ensure that specific attention was placed of those materials that were very mobile and those that have issues with bioaccumulation. This includes their behavior in humans as well as the environment. This also highlights the severity that the EU is attributing to this specific hazard class, which already meets the SVHC (substance of very high concern) criteria.
Another aspect of these new hazard classifications is the expanded requirements for testing of smaller volumes of imported product than had been required in the past. Testing protocols will now be required for import quantities of 1-10 MT and 10-100 MT. This is in addition to the traditional 100-1000 MT, 1,000-10,000 MT and 10,000+ MT
volume categories.
The EU is currently still lacking data on ED substances (as strange as it may sound) since only registered high volume imported substances have been tested up till now. This is bound to change, possibly soon, with new ED test requirements are bound to be included in the 1-10MT and 10-100MT test regimes under REACH.
The addition of these lower annual import volume quantities is likely to have a significant effect on the economic viability of certain imported products that are consumed in small quantities.
CW: What is the ECHA timeline for implementation of these new Classifications?
Rex: Figures 2 and 3 provide the ECHA (European Chemical Agency) timeline for reaching mandatory compliance with the revised regulations for the new hazard classifications and labeling require requirements.
Reach Regulation Revisions and Recent Changes
CW: How is REACH addressing sustainability?
Rex: Sustainability (within REACH) is one of the most far-reaching changes that is pending with the revision of the REACH Regulation. This effort is driven by public concerns regarding the potential adverse impacts of chemicals on the environment and human health. It is also elevated in importance by the political climate across the EU. The main objectives of an amendment to the REACH Regulation follows the 2020 “Chemicals Strategy for Sustainability” policy goals (CSS) (EC2020b).
CW: Can you elaborate on the current status of the sustainability initiative?
Rex: Consequently, the focus of this effort will be on measures for the targeted implementation of requirements for so-called “safe and sustainable by design chemicals.” It will also include the introduction of “non-toxic material cycles” and the establishment of an “essential use” concept. In addition, the most hazardous substances as well as endocrine disruptors and evaluation criteria for mixtures will be considered.
Studies commissioned in connection with the revision have largely been completed, whereby the evaluation of a reform of authorizations and restrictions as well as the support regarding the impact assessment are still open. After various delays, the draft of the intended amendments is expected in the course of this year.
The adoption of a corresponding proposal by the Commission is now expected for the last quarter of 2023 according to the Commission Work Programme for 2023 of 18.10.2022.
CW: What about biocides?
Rex: The European Biocidal Products Regulation (BPR) of September 2013 requires active biocide approval (Article 59 listing) followed by the authorization of the biocide product.
The process for approving new biocides has not changed since its inception in 2013 but new requirements for approving new biocides (e.g.. ED assessment) have been implemented in the meantime making it more complicated and costly.
The on-going active substance review program, which was targeted to end in 2024, will be prolonged as authorities are not able to assess the remaining “actives” within this timeframe. This is a “big deal” as they are now conducting the evaluations to complete the active chemicals permitted for use. Completion of this scheme will be pushed off
beyond 2024.
Once this list is set, its impact will be felt through restrictions on new biocide “actives,” which are to be approved first (the process currently taking many years) before marketing is allowed. The expense and time required to bring a new biocide to the market will be extremely high and likely prohibitive.
CW: What is the Candidate List of Substances for Authorization?
Rex: The REACH regulation maintains three separate lists with different requirements for the substances:
• The Candidate List (SVHC),
• Authorization (ANNEX XIV), and
• Restricted (Annex XVII)
The candidate list (SVHC) is the compilation of substances found to be carcinogenic, mutagenic or toxic to reproductive health through the “evaluation” phase of REACH. Any substance that is persistent, bio accumulative and toxic or is very persistent and very bio accumulative may also become an SVHC. SVHC’s are on the Candidate list because they have become candidates for Authorization, Annex XIV of the REACH regulation.
Note that the new CLP has new classifications for substances that are endocrine disruptors, persistent toxic, bio accumulative, or mobile. It is expected that the endocrine disruptor focus will place more substances on the list.
The Candidate list has been updated 29 times, the last being June 14, 2023, and currently contains 235 substances. See the link for a full listing of chemicals - Candidate List of substances of very high concern for Authorisation - ECHA (europa.eu). The REACH candidate list is the authentic version of chemicals under consideration.