Shiying Zheng, Robert Rasing and Nergiz Bozok, Evonik Corporation and Evonik Resource Efficiency11.05.18
Abstract
The coating industry is constantly facing the challenges of more stringent low emission requirements and fulfilling the need to improve productivity and reduce cost while maintaining high coating performance. Technology advancements equipped the industry with new waterborne epoxy systems that deliver fast cure speed, improved coating robustness and better aesthetics over the service life. Thanks to low volatile organic content (VOC) and excellent coating properties, waterborne epoxy coatings have become a commercially important technology and gained wide acceptance as environmentally friendly alternatives for solvent-borne and solvent-free epoxy systems.
This paper highlights the development of a new waterborne epoxy curing agent based on novel amine technology. The product provides extremely fast cure speed even under adverse conditions such as low temperature and high humidity; superior adhesion to substrates when used as a primer, particularly on damp concrete; and excellent aesthetics as a topcoat. This enables coating formulators to design new coating concepts such as a one-day floor system consisting of a primer and topcoat applied on the same day that delivers walk-on readiness the next morning. This paper discusses model formulations based on the new curing agent, key features, and benefits of the new waterborne system.
Background
Two-component epoxy systems are well-known for their excellent chemical resistance and mechanical properties as well as superior adhesion to a wide range of substrates. They have been widely used in concrete floor coatings and protective metal coatings. Driven by environmental and safety regulations, and workers health concerns, waterborne epoxy systems have become an important technology and gained wide acceptance in the coating industry. Key advantages of the waterborne systems span from low VOC, low emissions, low flammability, low toxicity, and easy clean up with water, to excellent adhesion even to a poorly prepared substrate.1 Technology advancements have equipped the industry with new waterborne epoxy systems that not only meet the more stringent low emission requirement but also fulfill the evolving market needs.
The key market drivers across the coating industry encompass minimizing downtime to improve cost and productivity, as well as improving coating robustness and better aesthetics over the service life. Minimizing downtime requires fast-cure coatings so that floor systems can be installed within a shorter time frame. However, fast cure often corresponds to short pot life or short working time.
Balancing fast cure and good working time has been a challenge for the industry for many years, both in solvent-borne and waterborne systems. Minimizing downtime can also be obtained by reducing the number of coatings in a floor system. Coatings that deliver improved robustness reduce the probability of installation failure and ultimately translate to lower cost per square meter.
Finally, better topcoat aesthetics aids to prolong the life cycle of an installed floor system. This paper describes a new waterborne epoxy system that can be used both as a primer and a topcoat, which delivers the balance of fast cure speed with good working time, and provides excellent adhesion to damp concrete substrates. Excellent aesthetics is yet another benefit.
This enables coating formulators to design new coating concepts such as a one-day floor system consisting of a primer and topcoat applied on the same day and ready to return to service the next day.
New Amine Building Block Design
We introduced the design of a new, fast-cure waterborne epoxy curing agent in a previous publication.2 The development was advanced in several stages. In the first stage of product development, a fast-cure non-waterborne system was developed. Secondly, the system was further modified to emulsify and cure liquid epoxy resin down to 10°C and high humidity, and form a film with high integrity. As explained, to obtain a fast cure speed, we leveraged a drying mechanism that is solely dependent on solvent evaporation referred to as ‘lacquer dry’, a concept that has been elegantly explained by Dubowik et.al. in the Waterborne Symposium edition of 1999.3 A conventional polyamide coating system, (hereafter abbreviated as “PA_SER”) composed of a solid epoxy resin (SER) and a viscous polyamide (ca. 400,000 cP), typically provides a dry-to-touch film after the solvent evaporates that is referred to as ‘lacquer dry’. Solvent evaporation is considered a much faster process so little chemical conversion has taken place when reaching a lacquer dry state.
Miller-Macosko Modeling
Conventional waterborne systems for concrete protection are mostly based on ‘liquid’ epoxy resin (LER), i.e., having a molecular weight of ca. 380 g/mol and a viscosity range of 10,000 to 15,000 cP at ambient temperature. By using a solid epoxy resin (SER) dispersion, we were expecting faster cure speed as a result of the higher molecular weight starting point. Similar to Dubowik et.al., we used the computer modeling of epoxy cure following Miller-Macosko calculations.3 We started with a preferred standard amine, isophorone diamine (IPD), for its good mechanical build and aesthetic properties and compared the results with conventional polyamides cured with LER and SER.
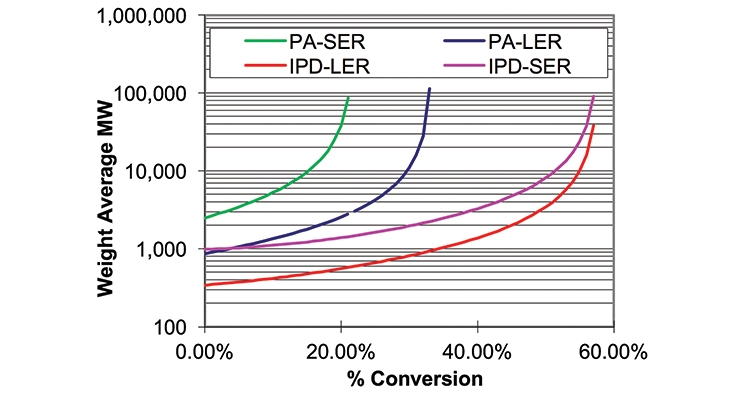
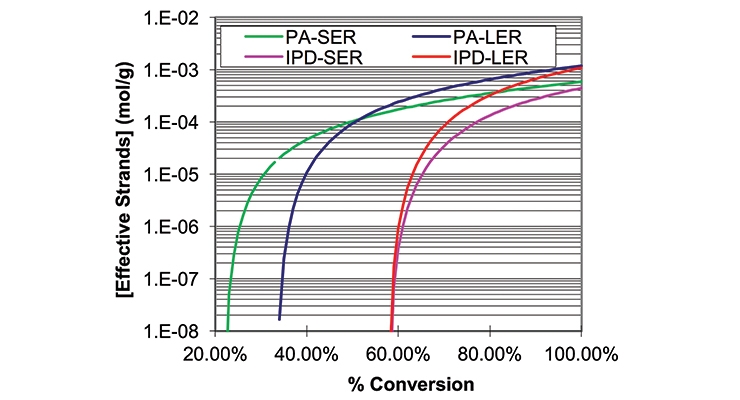
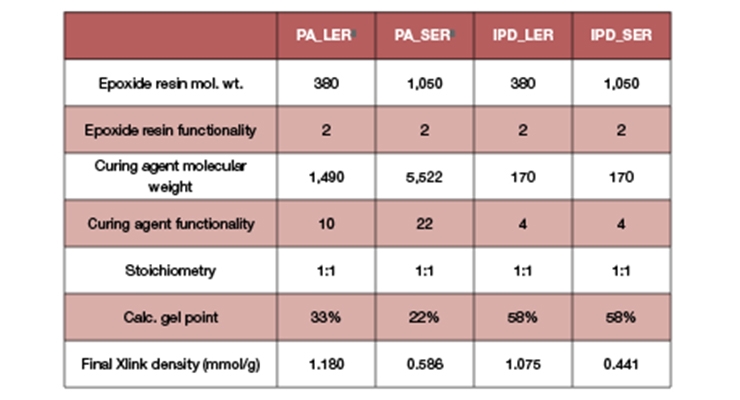
The data required for the calculations are summarized in Table 1, together with the calculated gel point and crosslink density expressed in millimoles per gram. Figure 1 shows a graphical representation of the weight average molecular weight (MW) and Figure 2 shows the concentrations of effective strands as a function of percent conversion. The latter can be interpreted as a mathematical expression of the crosslink density.
Since the conventional polyamide with SER (“PA_SER”) gives lacquer dry, we can assume that at 0% conversion the coating viscosity excluding its solvents would be higher than 10,000,000 cP.4
Not surprisingly, IPD with LER required substantial conversion (47%) to take place to reach the same lacquer dry type viscosity. This conversion required time and, as a result, the dry-to-touch times obtained were longer. In addition, the combination of IPD with SER still needed 33% conversion for a comparable level of molecular weight builds, again with longer cure times as a consequence.
Here, we also see a fundamental difference between SER in combination with a viscous polyamide and with IPD. While IPD with SER provided similar crosslink density as a viscous polyamide, the calculated gel point was at 58% conversion versus only 22% with PA_SER. Therefore, our aim to have a fast lacquer dry waterborne coating will need a faster cure co-amine in conjunction with IPD.
Typical amine curing agents can be categorized into aliphatic amines, cycloaliphatic amines, and (poly)amidoamines.5 Aliphatic amines, such as polyethylene amines, have high functionality and reactivity; on the other hand, they have a strong tendency to carbamation and exudation due to poor compatibility with epoxy resin. Cycloaliphatic amines such as IPD have excellent compatibility with epoxy resins due to the cycloaliphatic backbones, but have slower reactivity than aliphatics especially at low temperature. Good compatibility between the curing agent and epoxy resin leads to good coating appearance, good aesthetics and excellent intercoat adhesion. The challenge is to design a new amine building block that possesses both the high reactivity of an aliphatic amine and good resin compatibility of a cycloaliphatic amine. We have developed such a building block: a novel polyheterocyclic amine (Poly HCA) that delivers fast through-cure while maintaining good resin compatibility and excellent UV resistance. Poly HCA has low Gardner color and can be used as a sole curing agent or as a co-curing agent with amines. In order to have a good understanding of the unique properties demonstrated by Poly HCA, we carried out fundamental studies to monitor the epoxy cure process.
Fast Property Development of Poly HCA
The fast property development of Poly HCA was investigated by cure speed, viscosity build-up, and solvent resistance testing, such as methyl ethyl ketone (MEK) double rub. More in-depth studies were conducted to investigate the fundamental cure mechanism of Poly HCA by monitoring the curing process using near infrared and differential scanning calorimetry (DSC) analysis. Poly HCA was benchmarked against aliphatic amines, cycloaliphatic amines, and Mannich-base amine curing agents. For this, Poly HCA was tested with 40% benzyl alcohol and cured with standard LER (EEW=190) at 1:1 stoichiometry. The viscosity profiles were obtained on a Brookfield viscometer at 25°C using about 15 g of mixed material. Coatings for thin film set time and Persoz hardness were deposited on glass substrates at 150 µm wet film thickness. The thin film set time (TFST) was determined using a Beck-Koller recorder, in accordance with ASTM D5895. Persoz hardness was performed in accordance with ASTM D4366 after coatings were cured at 23°C or 10°C and 50% RH for 1, 2, and 7 days. MEK double rub testing was carried out according to ASTM 7835 on the samples based on the same cure schedule. Gloss was determined at an angle of 20 degree (20°) and 60 degree (60°) using a Gardner gloss meter according to ASTM D523. Measurements were made with the glass panel placed on a black cardboard background to minimize reflection. Shore D hardness was tested on ¼ inch thick clear casting in a circular metal lid with diameter of 2.75 inches using 35g of materials in accordance with the method described in ASTM D2240.
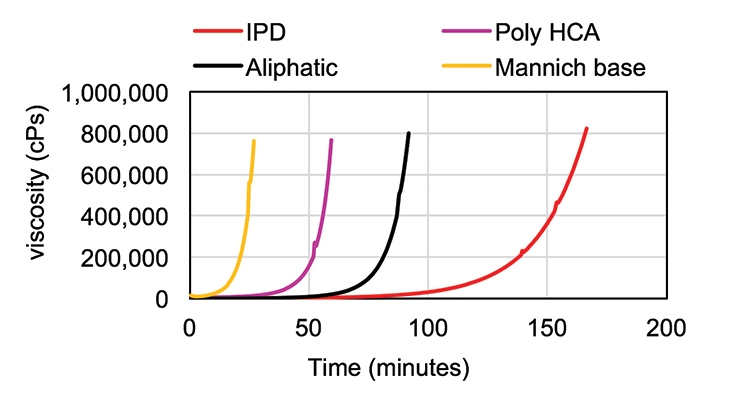
Figure 3 shows the comparison of cure viscosity profiles of Poly HCA with an aliphatic amine, cycloaliphatic IPD, and a Mannich-base curing agent. Poly HCA exhibited fast viscosity build-up with liquid epoxy resin, faster than the aliphatic amine and significantly faster than IPD, although it is slower than the Mannich base.
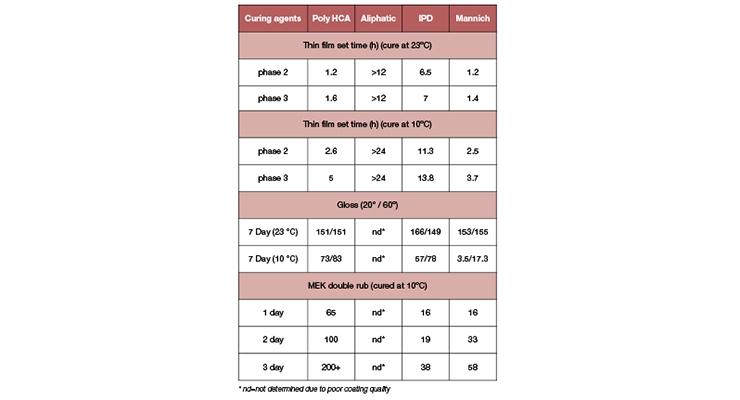
Fast viscosity build-up is the first indication of high reactivity. Test results in Table 2 clearly show that coatings based on Poly HCA also delivered fast dry speed at ambient and low temperatures.
The amine demonstrated significantly faster dry speed than the aliphatic amine and IPD, although slightly slower than the Mannich-base curing agent. The latter is typically used for fast low-temperature-cure epoxy systems. Poly HCA-based coatings also exhibit good coating appearance at low temperature, similar to cycloaliphatic IPD and substantially better than the Mannich-base curing agent. High gloss coating is indicative of good compatibility between resin and curing agent during the working time. Note that the aliphatic amine resulted in greasy and sweaty coatings, therefore making it difficult to obtain thin film set times, and gloss values. Poly HCA not only delivers fast dry speed but also shows good compatibility with resin.
Fast dry speed does not warrant high crosslinking density though. The MEK double rub test is an indicator of the through-cure properties of a film, and correlates to the degree of crosslinking. The higher the number of MEK double rubs, the better the MEK resistance, the greater the film integrity and the higher the crosslinking density. The low temperature 10°C MEK double rub data in Table 2 clearly demonstrated that Poly HCA offered coatings with good early through-cure at low temperature (1 and 2 days), significantly better than the benchmark curing agents. Although the Mannich-base curing agent provided fast dry speed, it did not offer good through-cure at low temperature after 7 days. Furthermore, the coating showed low gloss and poor appearance at low temperature, indicative of incompatibility with resin. The through-cure property can also be quantitatively monitored by the degree of cure using DSC.
Fundamental Studies of Fast Cure Mechanism of Poly HCA
1. DSC to monitor degree of cure and conversion.
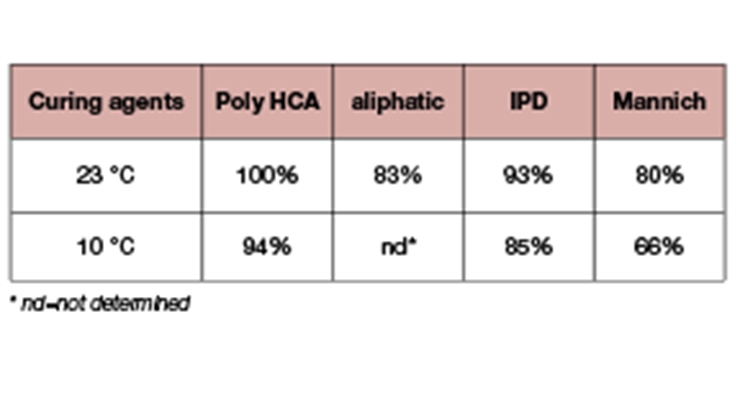
Fast property development has a correlation to the degree of reaction. The degree of reaction is directly translated to the degree of epoxy cure which can be measured by DSC.5 About 5~10mg epoxy-amine mixture sample was analyzed using a TA Instruments Q2000 DSC calibrated in T4P mode at a heating rate of 10°C/minute with Indium. The sample was heated from -50°C to 250°C at 10°C/minute, cooled back to -50°C and the test was repeated. The degree of cure was determined by subtracting the residual heat of cure after 7 days from the initial total heat of cure, divided by the initial total heat of cure. The results (Table 3) show that Poly HCA has a higher degree of cure both at 23°C and 10°C than the benchmark curing agents. This is likely due to the fast reactivity of Poly HCA. Aliphatic amine has a lower degree of cure at ambient temperature which can be attributed to the poor compatibility with epoxy resin. Although the Mannich base has fast dry speed and fast viscosity build up, it has a low degree of cure both at 23°C and 10°C. The results point to early vitrification preventing the system from reaching a higher degree of cure and this likely contributed to the low degree of crosslinking and a low number of MEK double rubs.
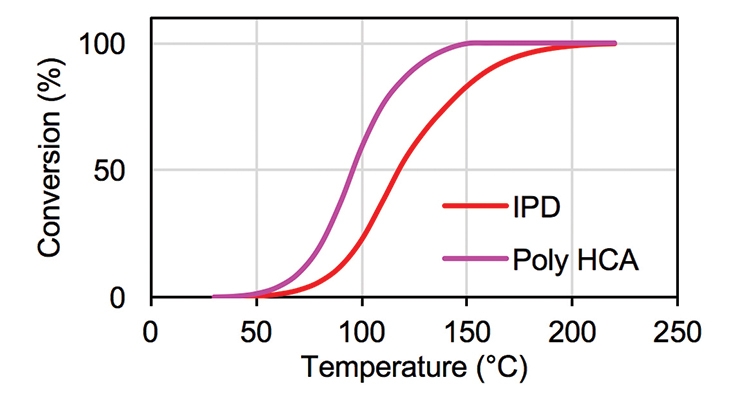
The cure conversion as a function of temperature can also be monitored by DSC. Figure 4 illustrates the conversion of Poly HCA and IPD as a function of temperature. The DSC was run at 10°C/minute. The conversion curves showed that at a given temperature, the amount of epoxy reacted with amine is substantially higher for Poly HCA than for IPD. The DSC method only gives the overall rate of reaction based on the assumption that the rate of the heat release is proportional to the rate of reaction. No information on individual species or functional groups can be obtained using DSC.
2. Near-infrared spectroscopy to monitor reaction conversion.
Fourier transform infrared spectroscopy (FTIR) spectroscopy not only can provide information on the individual species of interest such as epoxy and amine groups, but also monitors the cure reactions in real time. The functional groups of interest for epoxide cure reactions typically have well-isolated absorption bands in the near-infrared region of the spectrum (4000-10000 cm-1).
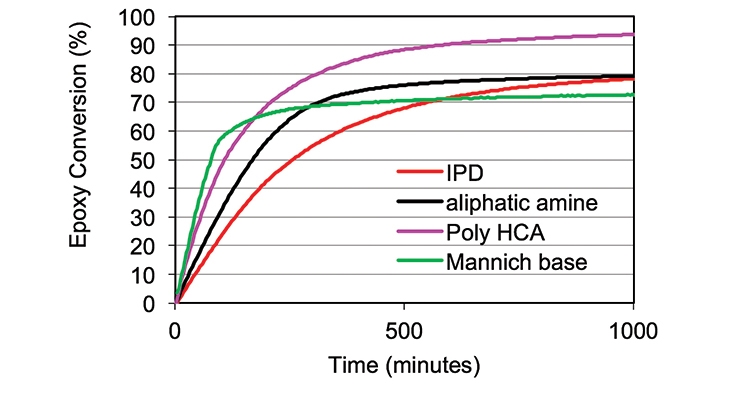
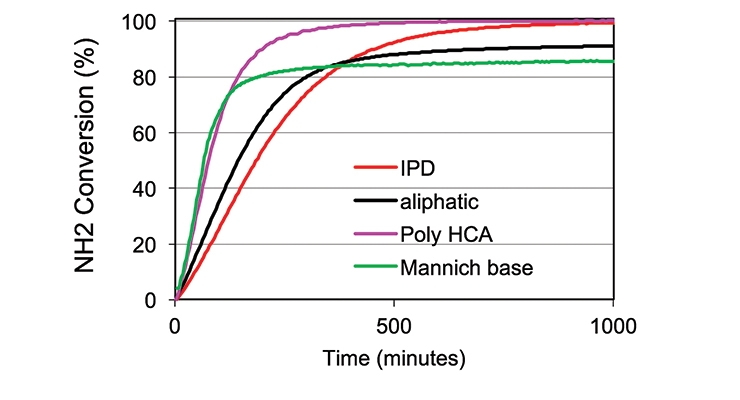
These factors make near-infrared FTIR an attractive analytical tool for the study of kinetics and mechanisms of epoxy resin cure reactions. As such, to delineate the reaction of functional epoxy and amine groups, near infrared FTIR was utilized to monitor the cure reaction and to semi-quantitatively determine the conversion of amine and epoxy groups presented in this paper.6 The conversion of oxirane (epoxy) and primary amine during the cure was monitored by the C-H stretch of the oxirane ring at 1646 nm and the N-H stretch of the primary amine at 2026 nm respectively. About 5 grams of the amine-epoxy mixture was mixed using FlackTeK DAC 250 SP SpeedMixer™ by Hauschild. After mixing, a small amount of sample was placed in a disposable sample cell of 0.8 mm path length and placed in an oven at 25°C. The NIR spectrometer used for the analysis was a Model 6500 near-infrared spectrometer equipped with an Interactance probe by Foss NIR Systems, Inc. The IR spectra were collected over about 24 hours, and the spectra analyzed using GRAMS software. Figures 3 and 4 show the conversion of epoxy and primary amine during the curing process, in comparison with IPD, an aliphatic amine, and a Mannich-base curative. Figures 5 and 6 clearly illustrate that Poly HCA has a higher conversion of the epoxy group and primary amine group than the benchmark amines. The result is consistent with the degree of cure and MEK double rub testing.
Yellowing Resistance of Poly HCA
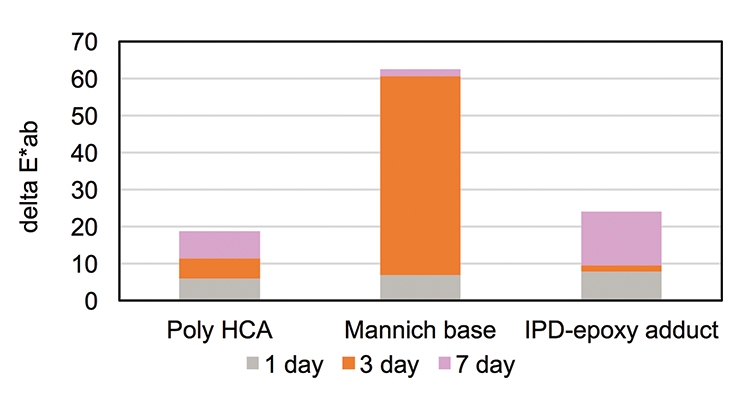
Figure 7 shows the comparison of epoxy coatings based on Poly HCA, a Mannich base and a conventional IPD-epoxy adduct curing agent. All coatings were prepared using standard bisphenol-A-based epoxy resin of (EEW 190) at 1:1 stoichiometry, applied at a wet film thickness of about 150 µm and cured at 23ºC and 50% relative humidity (RH) for 7 days. The coatings were then exposed to QUV A light, and the delta “yellowness index” was measured according to ASTM E313-10 under D65 illuminant at 1, 3, and 7 day UV light exposure.
Commonly used fast curing agents such as Mannich-base curing agents and accelerators for amine-cured epoxy coatings contribute to increased yellowing under UV light exposure. Figure 7 illustrates that Poly HCA overcomes the challenge and presents itself as a low-yellowing, fast-cure amine building block. The coating based on Poly HCA demonstrated comparable yellowing resistance as an IPD-epoxy adduct curing agent, and much better than the fast-cure Mannich base.
Poly HCA as a Fast Cure Co-amine in Combination with IPD
We have demonstrated that Poly HCA is a novel amine building block that delivers fast through-cure at low temperature. Commonly used fast curing agents such as Mannich-base curatives and accelerators contribute to increased yellowing of the coating under ultraviolet (UV) light exposure. We have reported in our previous publication2 that Poly HCA overcomes the challenge and presents itself as a low- yellowing fast-cure amine building block. The clear coating based on Poly HCA exhibited comparable yellowing resistance to a standard IPD-epoxy adduct curing agent, and much better than a Mannich-base curative.
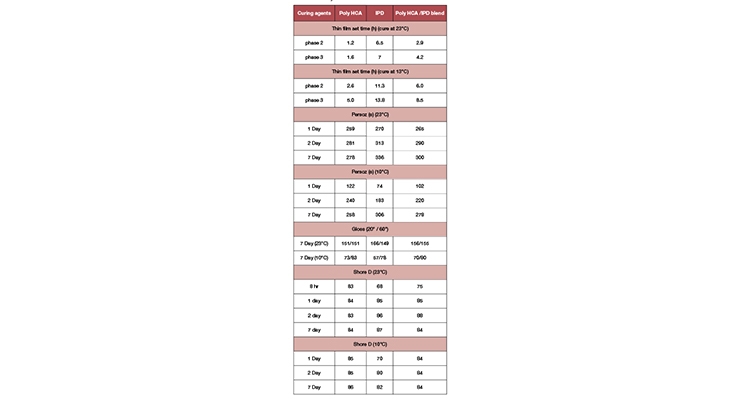
One objective of developing this amine building block is to design a fast-cure waterborne curing agent using IPD by taking advantage of IPD’s excellent mechanical property and good aesthetics. Our modeling studies showed that IPD alone with solid resin dispersion will not achieve the required fast lacquer dry. Per Table 4, Poly HCA combined with IPD showed accelerated cure and shorter thin film set times compared to IPD. It also provided improved early Persoz and shore D hardness development as well as higher gloss. The next step is to build this concept into the design of a fast-cure waterborne curing agent with IPD and Poly HCA.
New Fast Cure Waterborne Curing Agent
The demand for improved productivity has pushed for products that can be successfully applied at wider application windows, including wider temperature and humidity ranges, and multiple coats within one day. For concrete floor coatings, a minimum of two coats within one day is a desirable target: a primer and a topcoat. To fulfill such a one-day flooring concept, the critical element is to have a fast primer that allows a topcoat to be applied within a few hours by the same shift and same crew. We have developed a new waterborne curing agent, Prototype A, by rational design and incorporating the fast-cure amine building block Poly HCA discussed in this paper. Typical properties of the curing agent were reported in a previous publication.2 The curing agent was used in both primer and topcoat. A solid epoxy resin dispersion was used in the primer formulation for an ultra-fast cure at low temperature and high humidity. Using the same Prototype A, a diluted liquid epoxy resin was used in the topcoat formulation. While the SER dispersion offers fast lacquer dry, the LER provides good chemical resistance and mechanical properties in the topcoat. The differences in coating film formation in waterborne epoxies have been well documented.7 Detailed studies of Prototype A, model formulations, key features and benefits of the new waterborne system will be discussed next.
Primer Performance
1. Primer basic properties.
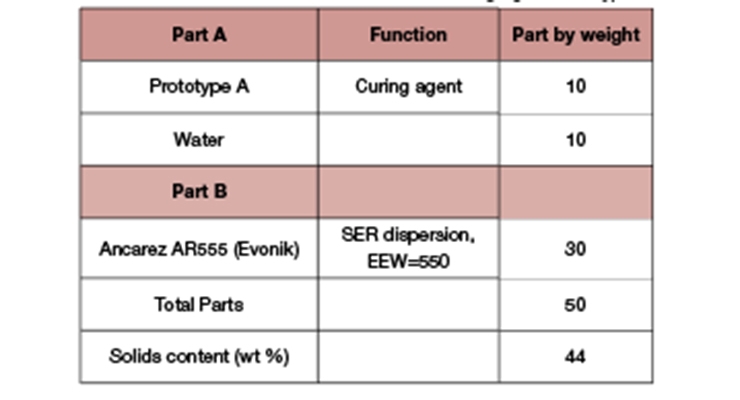
This section details the excellent performance of the primer using the new waterborne curing agent Prototype A, focusing on the adverse coating condition of low temperature and high humidity. The basic properties of Prototype A were reported previously.2 Table 5 shows the model primer formulation with a solid resin dispersion.
The primer formulation was evaluated for its performance at 10°C and 60% relative humidity. Water was added to Prototype A, then resin dispersion was added to the curing agent with mixing.
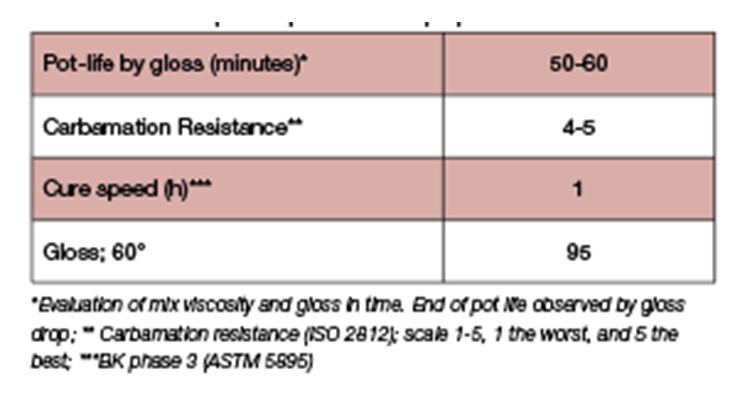
Clear coatings were applied on glass panels at 6 mil (150 µm) wet film thickness. Pot life was monitored by gloss stability, and drying time by a Beck-Koller recorder, in accordance with ASTM D5895. Carbamation resistance was assessed based on the method described in ISO 2812 and reported on a scale of 1-5 with 5 being the best. Table 6 demonstrates the primer performance at 10°C and 60% RH. The formulation provides a surprisingly good pot life of about one hour relative to its fast cure speed. Furthermore, the coating exhibited high gloss and excellent resistance to carbamation and water spotting, the latter being critical to ensure good intercoat adhesion to the subsequent layer.
The advantageous feature of the primer formulation shown in Table 5 is its ease of use in the field. It was formulated as a two-component system by pre-diluting with the desired amount of water.
The pre-diluted primer formulation showed no phase separation or changes in performance up to one month at ambient temperature. Longer storage times and conditions are under evaluation.
2. Primer dry time and adhesion to concrete.
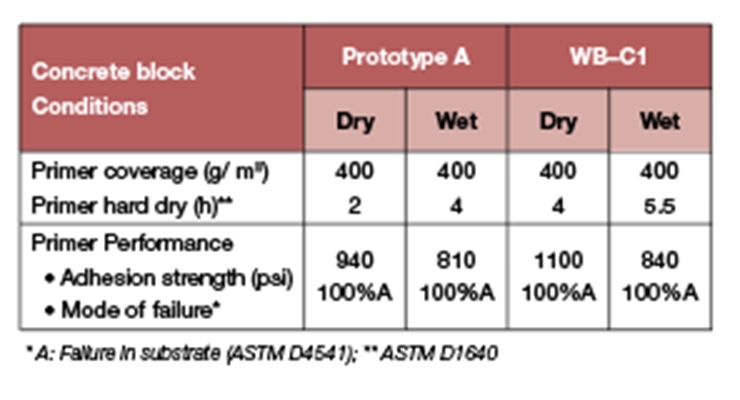
The new primer formulation shown in Table 5 was evaluated for dry time and adhesion to wet and dry concrete and compared to a commercially available fast waterborne curing agent (WB-C1).
The B25 concrete blocks (EN206-1, class C25/30) used for the adhesion testing were first cleaned by wire brush, followed by vacuuming to remove the loose concrete dust. The blocks were acclimatized at the test temperature for at least 24 hours. The blocks for wet concrete testing were kept submerged in water during the acclimatization period. The blocks were then removed from water and excess surface water wiped off prior to applying a primer. To ensure the concrete block was properly acclimated to the designated temperature and moisture content, actual temperature and moisture were monitored before applying the primer. The temperature of all blocks was determined using an infrared thermometer, and the relative moisture content was determined by a Testo 606-1 material moisture meter that was set to material number 3 (cement screed, concrete). Primers were applied onto concrete by brush to the target grams per square meter. For wet adhesion tests, once the primers were applied, the concrete blocks were placed in a container with ridges on the bottom allowing water to circulate underneath the blocks. Water, at the test temperature, was added until reaching 1 centimeter below the surface and maintained at that level during the test.
After primer application, the drying time was determined by thumb twist testing according to ASTM 1640. The primer was cured at the designated temperature and humidity for seven days before adhesion testing (ASTM D4541) was carried out. Dollies were glued to the surface and the glue was cured overnight. Dollies were then pulled off using a PAT-adhesion tester. Both the tensile stress at break and the type of failure were recorded. Adhesion tests were carried out in triplicate.
Table 7 summarizes the primer dry times and adhesion to dry and wet concrete. Prototype A gave excellent adhesion to wet and dry concrete, comparable to the commercial product WB-C1 while providing faster dry times.
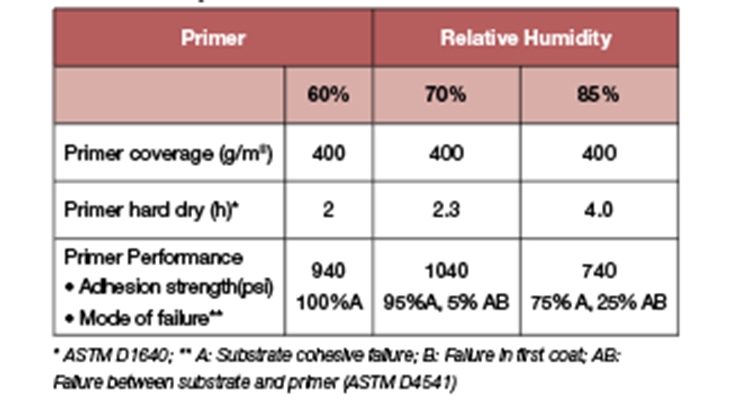
In general, for waterborne systems, the relative humidity can have a significant impact on drying times and adhesion strength of the coating. High humidity prevents water from evaporating and slows down the cure speed of a coating. Data presented in Table 8 illustrates the characteristics of Prototype A. Even at high humidity, the primer continued to deliver dry speeds of 4 hours. With the increase of humidity to 85%, only 25% loss in cohesive adhesion was observed. The fast drying primer based on Prototype A enables the applicators to apply the floor system in one day by providing sufficient time to apply the topcoat even at 85% humidity.
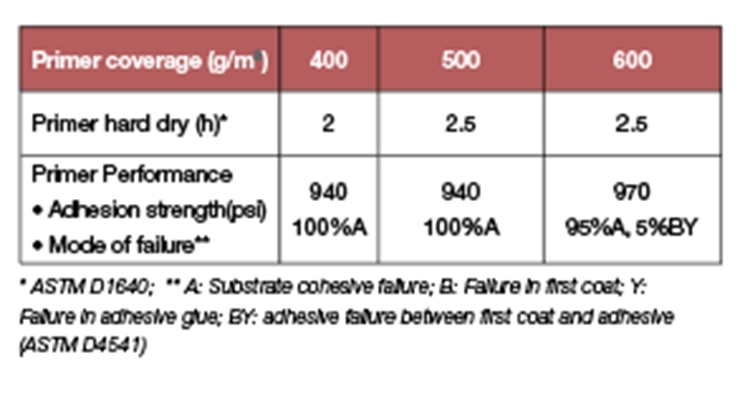
In addition to the fast drying and excellent adhesion to concrete, another key feature of primers based on Prototype A is the high film build up to about 23.5 mil (600 µm). Results in Table 9 clearly show the retention of drying time and adhesion at higher film builds. Typically in waterborne systems, as the coating thickness increases, it is more difficult for water to evaporate from the coating, especially at low temperature and high humidity. The end result is a longer drying time and entrapped water inside the coating that leads to poor coating appearance and inferior coating properties.
3. Primer overcoatability.
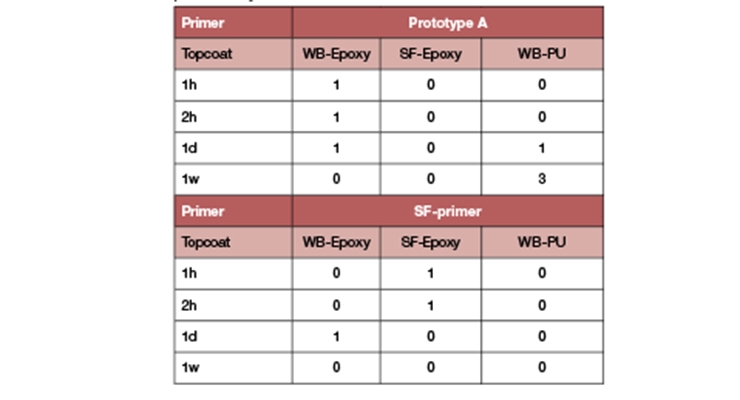
Overcoatability and adhesion strength between primer and topcoat is yet another key requirement to meet. Overcoatability of the primer formulation in Table 5 (Prototype A) was evaluated by cross hatch testing and compared to a solvent-free 2K epoxy primer that used a standard cycloaliphatic amine curing agent (SF primer). Primers were topcoated with a waterborne polyurethane (WB-PU), a white pigmented topcoat based on Prototype A (WB-Epoxy), and a grey pigmented topcoat based on a solvent-free 2K epoxy (SF-Epoxy). The top coats were applied at 400 g/m2 and directly after the primer was dry-to-touch (thumb twist, ASTM D1640). Subsequently, the test was repeated after 1 hour, 2 hours, 1 day, and 1 week after the dry-to-touch time. After the topcoats were cured for 7 days, the systems were subjected to the cross-hatch adhesion test (ISO 2409, rating 1 (excellent adhesion) to 5 (full delamination). The results (Table 10) showed excellent overcoatability. The results were similar to the solvent-free 2K epoxy primer. Longer overcoat windows are the subject of further study.
One-Day Flooring System
Topcoat Performance
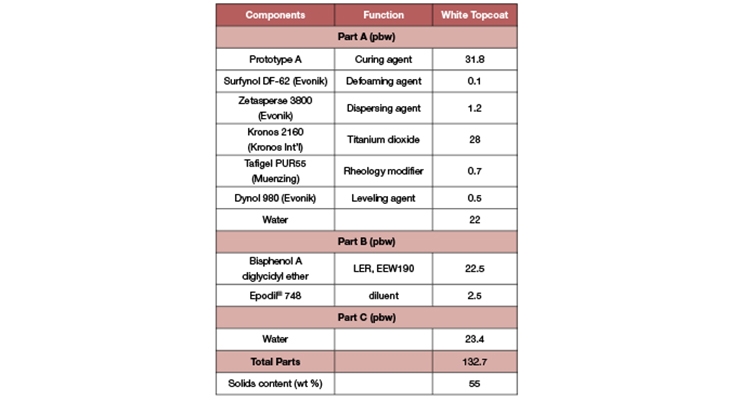
We have detailed the properties and excellent performance of the primer formulation based on the new waterborne curing agent Prototype A. In this section, we are focusing on the performance attribute and the key benefits of the flooring system using Prototype A as the topcoat. For this, Prototype A was formulated with diluted liquid epoxy resin in a model topcoat formulation (Table 11).
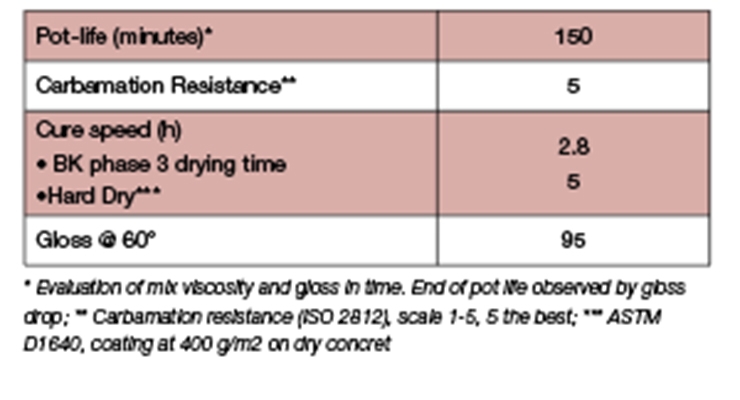
Basic performance properties of the white paint formulation cured at 10°C/60% RH are summarized in Table 12. Results clearly show the fast dry property of the topcoat formulation. Combining Prototype A in the primer and topcoat will give applicators a solution for a one-day flooring concept.
Intercoat Adhesion Between Primer and Topcoat
As discussed in previous sections, intercoat adhesion between primer and topcoat is critical for the longevity of floor systems. The intercoat adhesion of a floor system formulated with Prototype A in both the primer (Table 5) and the topcoat (Table 11) was evaluated on dry and wet concrete under adverse conditions. The results are summarized in Table 13. The floor system exhibited excellent intercoat adhesion and delivered a walk-on time of less than
24 hours, even on wet concrete conditions. Although using diluted LER in the topcoat lengthened the drying times on wet concrete, it will continue to allow formulators to design a floor system with a return to service after 1 day cure under adverse conditions of low temperature and high humidity.
UV-Resistance of the Floor System
The UV-resistance of topcoats was evaluated by an accelerated UV exposure test. The solvent-free epoxy system chosen is known for its high UV durability feature. Coatings were applied on S36i steel Q-panels at 6 mil (150 µm) wet film thickness and cured for seven days at 23 °C /50% RH. The panels were then placed into a QUV accelerated weathering tester set to a test temperature of 45°C and an irradiance of 0.89 W/m2/nm (UV mode only). Delta E and yellowing index data were obtained over the 250 hour time interval.
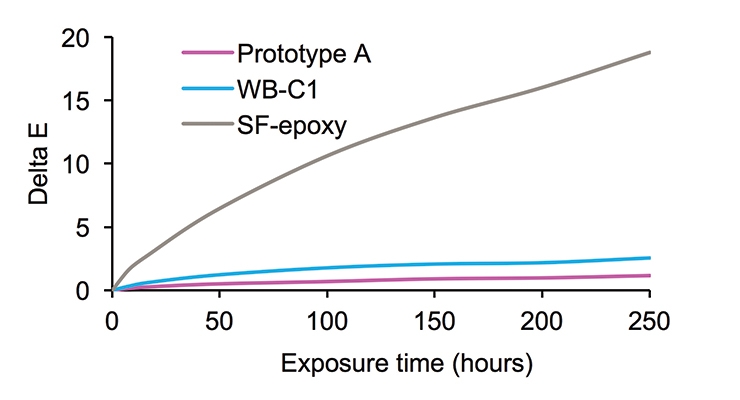
Figure 8 exemplifies the very good UV-resistance feature of the new waterborne Prototype A. After 250 hours of UV exposure, a delta E is only one, compared to a delta E of 19 for the SF-epoxy. Prototype A performed marginally better than WB-C1 with a delta E of 2.5.
Conclusions
We have developed a new yellowing-resistant, fast-cure amine building block (Poly HCA) that can be incorporated into a waterborne curing agent. Fundamental studies of Poly HCA shed light on the fast cure property. Poly HCA exhibits an ultra-fast through-cure property that is manifested in high epoxy and amine conversion, thus resulting in high degree of cure. The combination of using IPD with Poly HCA in a new waterborne curing agent, Prototype A, offers a unique solution. The product possesses fast cure speed even under adverse conditions, excellent aesthetics, and outstanding UV resistance. It enables coating formulators to design new floor systems consisting of a primer and a topcoat applied on the same day, and that delivers the fast return to service the next morning. As such, waterborne coating technology continues to meet stringent market requirements both in terms of performance and environmental demands.
Acknowledgement
The authors would like to thank colleagues Mike Oberlander, Daniel Bleumink and Edwin Lijffijt for carrying out the experimental work.
References
1. Richardson F. Pigment and Resin Tech., 1973, 5, 41-43.
2. Zheng, S., Rasing, R. and Bozok, N. Waterborne Symposium, New Orleans, 2018, in press.
3. D.A. Dubowik, F.H. Walker and W.E. Starner, Novel curing agent technology for high solids epoxy coatings, International Waterborne, High Solids, and Powder Coatings Symposium, New Orleans, February 1999.
4. (a) Miller, D.R. and Macosko, C.W. Macromolecules, 1976, 9, 207; b) Miller, D.R., Valles, E.M. and Macosko, C.W. Polym. Eng. Sci., 1979, 19 (4), 272.
5. (a) Lee, W. I., Loos, A. C., Springer, G. S. J. Composite Mat. 1982, 16, 510. Weinmann, D.J., (b) Dangayach, K., Smith, C. Journal of Coatings Technology, 1996, 68 (863), 29-37
6. Xu, L.; Fu, J. H.; Schlup, J. R. J. Am. Chem. Soc. 1994, 116, 2821-2826; Fu, J. H.; Schlup, J. R. J. Appl. Polymer Sci. 1993, 49, 219-227
7. (a) Wegmann, A. Prog. Org. Coatings 1997, 32, 231-239 (b) Walker, F. H. and Schaffer, O.; “Film Formation and Morphology in Two-Component, Ambient-Cured, Waterborne Epoxy Coatings” in Provder, T., Ed. Film Formation, ACS Symposium Series 648, American Chemical Society: Washington D. C., 1996, page 403-417.
The coating industry is constantly facing the challenges of more stringent low emission requirements and fulfilling the need to improve productivity and reduce cost while maintaining high coating performance. Technology advancements equipped the industry with new waterborne epoxy systems that deliver fast cure speed, improved coating robustness and better aesthetics over the service life. Thanks to low volatile organic content (VOC) and excellent coating properties, waterborne epoxy coatings have become a commercially important technology and gained wide acceptance as environmentally friendly alternatives for solvent-borne and solvent-free epoxy systems.
This paper highlights the development of a new waterborne epoxy curing agent based on novel amine technology. The product provides extremely fast cure speed even under adverse conditions such as low temperature and high humidity; superior adhesion to substrates when used as a primer, particularly on damp concrete; and excellent aesthetics as a topcoat. This enables coating formulators to design new coating concepts such as a one-day floor system consisting of a primer and topcoat applied on the same day that delivers walk-on readiness the next morning. This paper discusses model formulations based on the new curing agent, key features, and benefits of the new waterborne system.
Background
Two-component epoxy systems are well-known for their excellent chemical resistance and mechanical properties as well as superior adhesion to a wide range of substrates. They have been widely used in concrete floor coatings and protective metal coatings. Driven by environmental and safety regulations, and workers health concerns, waterborne epoxy systems have become an important technology and gained wide acceptance in the coating industry. Key advantages of the waterborne systems span from low VOC, low emissions, low flammability, low toxicity, and easy clean up with water, to excellent adhesion even to a poorly prepared substrate.1 Technology advancements have equipped the industry with new waterborne epoxy systems that not only meet the more stringent low emission requirement but also fulfill the evolving market needs.
The key market drivers across the coating industry encompass minimizing downtime to improve cost and productivity, as well as improving coating robustness and better aesthetics over the service life. Minimizing downtime requires fast-cure coatings so that floor systems can be installed within a shorter time frame. However, fast cure often corresponds to short pot life or short working time.
Balancing fast cure and good working time has been a challenge for the industry for many years, both in solvent-borne and waterborne systems. Minimizing downtime can also be obtained by reducing the number of coatings in a floor system. Coatings that deliver improved robustness reduce the probability of installation failure and ultimately translate to lower cost per square meter.
Finally, better topcoat aesthetics aids to prolong the life cycle of an installed floor system. This paper describes a new waterborne epoxy system that can be used both as a primer and a topcoat, which delivers the balance of fast cure speed with good working time, and provides excellent adhesion to damp concrete substrates. Excellent aesthetics is yet another benefit.
This enables coating formulators to design new coating concepts such as a one-day floor system consisting of a primer and topcoat applied on the same day and ready to return to service the next day.
New Amine Building Block Design
We introduced the design of a new, fast-cure waterborne epoxy curing agent in a previous publication.2 The development was advanced in several stages. In the first stage of product development, a fast-cure non-waterborne system was developed. Secondly, the system was further modified to emulsify and cure liquid epoxy resin down to 10°C and high humidity, and form a film with high integrity. As explained, to obtain a fast cure speed, we leveraged a drying mechanism that is solely dependent on solvent evaporation referred to as ‘lacquer dry’, a concept that has been elegantly explained by Dubowik et.al. in the Waterborne Symposium edition of 1999.3 A conventional polyamide coating system, (hereafter abbreviated as “PA_SER”) composed of a solid epoxy resin (SER) and a viscous polyamide (ca. 400,000 cP), typically provides a dry-to-touch film after the solvent evaporates that is referred to as ‘lacquer dry’. Solvent evaporation is considered a much faster process so little chemical conversion has taken place when reaching a lacquer dry state.
Miller-Macosko Modeling
Conventional waterborne systems for concrete protection are mostly based on ‘liquid’ epoxy resin (LER), i.e., having a molecular weight of ca. 380 g/mol and a viscosity range of 10,000 to 15,000 cP at ambient temperature. By using a solid epoxy resin (SER) dispersion, we were expecting faster cure speed as a result of the higher molecular weight starting point. Similar to Dubowik et.al., we used the computer modeling of epoxy cure following Miller-Macosko calculations.3 We started with a preferred standard amine, isophorone diamine (IPD), for its good mechanical build and aesthetic properties and compared the results with conventional polyamides cured with LER and SER.
The data required for the calculations are summarized in Table 1, together with the calculated gel point and crosslink density expressed in millimoles per gram. Figure 1 shows a graphical representation of the weight average molecular weight (MW) and Figure 2 shows the concentrations of effective strands as a function of percent conversion. The latter can be interpreted as a mathematical expression of the crosslink density.
Since the conventional polyamide with SER (“PA_SER”) gives lacquer dry, we can assume that at 0% conversion the coating viscosity excluding its solvents would be higher than 10,000,000 cP.4
Not surprisingly, IPD with LER required substantial conversion (47%) to take place to reach the same lacquer dry type viscosity. This conversion required time and, as a result, the dry-to-touch times obtained were longer. In addition, the combination of IPD with SER still needed 33% conversion for a comparable level of molecular weight builds, again with longer cure times as a consequence.
Here, we also see a fundamental difference between SER in combination with a viscous polyamide and with IPD. While IPD with SER provided similar crosslink density as a viscous polyamide, the calculated gel point was at 58% conversion versus only 22% with PA_SER. Therefore, our aim to have a fast lacquer dry waterborne coating will need a faster cure co-amine in conjunction with IPD.
Typical amine curing agents can be categorized into aliphatic amines, cycloaliphatic amines, and (poly)amidoamines.5 Aliphatic amines, such as polyethylene amines, have high functionality and reactivity; on the other hand, they have a strong tendency to carbamation and exudation due to poor compatibility with epoxy resin. Cycloaliphatic amines such as IPD have excellent compatibility with epoxy resins due to the cycloaliphatic backbones, but have slower reactivity than aliphatics especially at low temperature. Good compatibility between the curing agent and epoxy resin leads to good coating appearance, good aesthetics and excellent intercoat adhesion. The challenge is to design a new amine building block that possesses both the high reactivity of an aliphatic amine and good resin compatibility of a cycloaliphatic amine. We have developed such a building block: a novel polyheterocyclic amine (Poly HCA) that delivers fast through-cure while maintaining good resin compatibility and excellent UV resistance. Poly HCA has low Gardner color and can be used as a sole curing agent or as a co-curing agent with amines. In order to have a good understanding of the unique properties demonstrated by Poly HCA, we carried out fundamental studies to monitor the epoxy cure process.
Fast Property Development of Poly HCA
The fast property development of Poly HCA was investigated by cure speed, viscosity build-up, and solvent resistance testing, such as methyl ethyl ketone (MEK) double rub. More in-depth studies were conducted to investigate the fundamental cure mechanism of Poly HCA by monitoring the curing process using near infrared and differential scanning calorimetry (DSC) analysis. Poly HCA was benchmarked against aliphatic amines, cycloaliphatic amines, and Mannich-base amine curing agents. For this, Poly HCA was tested with 40% benzyl alcohol and cured with standard LER (EEW=190) at 1:1 stoichiometry. The viscosity profiles were obtained on a Brookfield viscometer at 25°C using about 15 g of mixed material. Coatings for thin film set time and Persoz hardness were deposited on glass substrates at 150 µm wet film thickness. The thin film set time (TFST) was determined using a Beck-Koller recorder, in accordance with ASTM D5895. Persoz hardness was performed in accordance with ASTM D4366 after coatings were cured at 23°C or 10°C and 50% RH for 1, 2, and 7 days. MEK double rub testing was carried out according to ASTM 7835 on the samples based on the same cure schedule. Gloss was determined at an angle of 20 degree (20°) and 60 degree (60°) using a Gardner gloss meter according to ASTM D523. Measurements were made with the glass panel placed on a black cardboard background to minimize reflection. Shore D hardness was tested on ¼ inch thick clear casting in a circular metal lid with diameter of 2.75 inches using 35g of materials in accordance with the method described in ASTM D2240.
Figure 3 shows the comparison of cure viscosity profiles of Poly HCA with an aliphatic amine, cycloaliphatic IPD, and a Mannich-base curing agent. Poly HCA exhibited fast viscosity build-up with liquid epoxy resin, faster than the aliphatic amine and significantly faster than IPD, although it is slower than the Mannich base.
Fast viscosity build-up is the first indication of high reactivity. Test results in Table 2 clearly show that coatings based on Poly HCA also delivered fast dry speed at ambient and low temperatures.
The amine demonstrated significantly faster dry speed than the aliphatic amine and IPD, although slightly slower than the Mannich-base curing agent. The latter is typically used for fast low-temperature-cure epoxy systems. Poly HCA-based coatings also exhibit good coating appearance at low temperature, similar to cycloaliphatic IPD and substantially better than the Mannich-base curing agent. High gloss coating is indicative of good compatibility between resin and curing agent during the working time. Note that the aliphatic amine resulted in greasy and sweaty coatings, therefore making it difficult to obtain thin film set times, and gloss values. Poly HCA not only delivers fast dry speed but also shows good compatibility with resin.
Fast dry speed does not warrant high crosslinking density though. The MEK double rub test is an indicator of the through-cure properties of a film, and correlates to the degree of crosslinking. The higher the number of MEK double rubs, the better the MEK resistance, the greater the film integrity and the higher the crosslinking density. The low temperature 10°C MEK double rub data in Table 2 clearly demonstrated that Poly HCA offered coatings with good early through-cure at low temperature (1 and 2 days), significantly better than the benchmark curing agents. Although the Mannich-base curing agent provided fast dry speed, it did not offer good through-cure at low temperature after 7 days. Furthermore, the coating showed low gloss and poor appearance at low temperature, indicative of incompatibility with resin. The through-cure property can also be quantitatively monitored by the degree of cure using DSC.
Fundamental Studies of Fast Cure Mechanism of Poly HCA
1. DSC to monitor degree of cure and conversion.
Fast property development has a correlation to the degree of reaction. The degree of reaction is directly translated to the degree of epoxy cure which can be measured by DSC.5 About 5~10mg epoxy-amine mixture sample was analyzed using a TA Instruments Q2000 DSC calibrated in T4P mode at a heating rate of 10°C/minute with Indium. The sample was heated from -50°C to 250°C at 10°C/minute, cooled back to -50°C and the test was repeated. The degree of cure was determined by subtracting the residual heat of cure after 7 days from the initial total heat of cure, divided by the initial total heat of cure. The results (Table 3) show that Poly HCA has a higher degree of cure both at 23°C and 10°C than the benchmark curing agents. This is likely due to the fast reactivity of Poly HCA. Aliphatic amine has a lower degree of cure at ambient temperature which can be attributed to the poor compatibility with epoxy resin. Although the Mannich base has fast dry speed and fast viscosity build up, it has a low degree of cure both at 23°C and 10°C. The results point to early vitrification preventing the system from reaching a higher degree of cure and this likely contributed to the low degree of crosslinking and a low number of MEK double rubs.
The cure conversion as a function of temperature can also be monitored by DSC. Figure 4 illustrates the conversion of Poly HCA and IPD as a function of temperature. The DSC was run at 10°C/minute. The conversion curves showed that at a given temperature, the amount of epoxy reacted with amine is substantially higher for Poly HCA than for IPD. The DSC method only gives the overall rate of reaction based on the assumption that the rate of the heat release is proportional to the rate of reaction. No information on individual species or functional groups can be obtained using DSC.
2. Near-infrared spectroscopy to monitor reaction conversion.
Fourier transform infrared spectroscopy (FTIR) spectroscopy not only can provide information on the individual species of interest such as epoxy and amine groups, but also monitors the cure reactions in real time. The functional groups of interest for epoxide cure reactions typically have well-isolated absorption bands in the near-infrared region of the spectrum (4000-10000 cm-1).
These factors make near-infrared FTIR an attractive analytical tool for the study of kinetics and mechanisms of epoxy resin cure reactions. As such, to delineate the reaction of functional epoxy and amine groups, near infrared FTIR was utilized to monitor the cure reaction and to semi-quantitatively determine the conversion of amine and epoxy groups presented in this paper.6 The conversion of oxirane (epoxy) and primary amine during the cure was monitored by the C-H stretch of the oxirane ring at 1646 nm and the N-H stretch of the primary amine at 2026 nm respectively. About 5 grams of the amine-epoxy mixture was mixed using FlackTeK DAC 250 SP SpeedMixer™ by Hauschild. After mixing, a small amount of sample was placed in a disposable sample cell of 0.8 mm path length and placed in an oven at 25°C. The NIR spectrometer used for the analysis was a Model 6500 near-infrared spectrometer equipped with an Interactance probe by Foss NIR Systems, Inc. The IR spectra were collected over about 24 hours, and the spectra analyzed using GRAMS software. Figures 3 and 4 show the conversion of epoxy and primary amine during the curing process, in comparison with IPD, an aliphatic amine, and a Mannich-base curative. Figures 5 and 6 clearly illustrate that Poly HCA has a higher conversion of the epoxy group and primary amine group than the benchmark amines. The result is consistent with the degree of cure and MEK double rub testing.
Yellowing Resistance of Poly HCA
Figure 7 shows the comparison of epoxy coatings based on Poly HCA, a Mannich base and a conventional IPD-epoxy adduct curing agent. All coatings were prepared using standard bisphenol-A-based epoxy resin of (EEW 190) at 1:1 stoichiometry, applied at a wet film thickness of about 150 µm and cured at 23ºC and 50% relative humidity (RH) for 7 days. The coatings were then exposed to QUV A light, and the delta “yellowness index” was measured according to ASTM E313-10 under D65 illuminant at 1, 3, and 7 day UV light exposure.
Commonly used fast curing agents such as Mannich-base curing agents and accelerators for amine-cured epoxy coatings contribute to increased yellowing under UV light exposure. Figure 7 illustrates that Poly HCA overcomes the challenge and presents itself as a low-yellowing, fast-cure amine building block. The coating based on Poly HCA demonstrated comparable yellowing resistance as an IPD-epoxy adduct curing agent, and much better than the fast-cure Mannich base.
Poly HCA as a Fast Cure Co-amine in Combination with IPD
We have demonstrated that Poly HCA is a novel amine building block that delivers fast through-cure at low temperature. Commonly used fast curing agents such as Mannich-base curatives and accelerators contribute to increased yellowing of the coating under ultraviolet (UV) light exposure. We have reported in our previous publication2 that Poly HCA overcomes the challenge and presents itself as a low- yellowing fast-cure amine building block. The clear coating based on Poly HCA exhibited comparable yellowing resistance to a standard IPD-epoxy adduct curing agent, and much better than a Mannich-base curative.
One objective of developing this amine building block is to design a fast-cure waterborne curing agent using IPD by taking advantage of IPD’s excellent mechanical property and good aesthetics. Our modeling studies showed that IPD alone with solid resin dispersion will not achieve the required fast lacquer dry. Per Table 4, Poly HCA combined with IPD showed accelerated cure and shorter thin film set times compared to IPD. It also provided improved early Persoz and shore D hardness development as well as higher gloss. The next step is to build this concept into the design of a fast-cure waterborne curing agent with IPD and Poly HCA.
New Fast Cure Waterborne Curing Agent
The demand for improved productivity has pushed for products that can be successfully applied at wider application windows, including wider temperature and humidity ranges, and multiple coats within one day. For concrete floor coatings, a minimum of two coats within one day is a desirable target: a primer and a topcoat. To fulfill such a one-day flooring concept, the critical element is to have a fast primer that allows a topcoat to be applied within a few hours by the same shift and same crew. We have developed a new waterborne curing agent, Prototype A, by rational design and incorporating the fast-cure amine building block Poly HCA discussed in this paper. Typical properties of the curing agent were reported in a previous publication.2 The curing agent was used in both primer and topcoat. A solid epoxy resin dispersion was used in the primer formulation for an ultra-fast cure at low temperature and high humidity. Using the same Prototype A, a diluted liquid epoxy resin was used in the topcoat formulation. While the SER dispersion offers fast lacquer dry, the LER provides good chemical resistance and mechanical properties in the topcoat. The differences in coating film formation in waterborne epoxies have been well documented.7 Detailed studies of Prototype A, model formulations, key features and benefits of the new waterborne system will be discussed next.
Primer Performance
1. Primer basic properties.
This section details the excellent performance of the primer using the new waterborne curing agent Prototype A, focusing on the adverse coating condition of low temperature and high humidity. The basic properties of Prototype A were reported previously.2 Table 5 shows the model primer formulation with a solid resin dispersion.
The primer formulation was evaluated for its performance at 10°C and 60% relative humidity. Water was added to Prototype A, then resin dispersion was added to the curing agent with mixing.
Clear coatings were applied on glass panels at 6 mil (150 µm) wet film thickness. Pot life was monitored by gloss stability, and drying time by a Beck-Koller recorder, in accordance with ASTM D5895. Carbamation resistance was assessed based on the method described in ISO 2812 and reported on a scale of 1-5 with 5 being the best. Table 6 demonstrates the primer performance at 10°C and 60% RH. The formulation provides a surprisingly good pot life of about one hour relative to its fast cure speed. Furthermore, the coating exhibited high gloss and excellent resistance to carbamation and water spotting, the latter being critical to ensure good intercoat adhesion to the subsequent layer.
The advantageous feature of the primer formulation shown in Table 5 is its ease of use in the field. It was formulated as a two-component system by pre-diluting with the desired amount of water.
The pre-diluted primer formulation showed no phase separation or changes in performance up to one month at ambient temperature. Longer storage times and conditions are under evaluation.
2. Primer dry time and adhesion to concrete.
The new primer formulation shown in Table 5 was evaluated for dry time and adhesion to wet and dry concrete and compared to a commercially available fast waterborne curing agent (WB-C1).
The B25 concrete blocks (EN206-1, class C25/30) used for the adhesion testing were first cleaned by wire brush, followed by vacuuming to remove the loose concrete dust. The blocks were acclimatized at the test temperature for at least 24 hours. The blocks for wet concrete testing were kept submerged in water during the acclimatization period. The blocks were then removed from water and excess surface water wiped off prior to applying a primer. To ensure the concrete block was properly acclimated to the designated temperature and moisture content, actual temperature and moisture were monitored before applying the primer. The temperature of all blocks was determined using an infrared thermometer, and the relative moisture content was determined by a Testo 606-1 material moisture meter that was set to material number 3 (cement screed, concrete). Primers were applied onto concrete by brush to the target grams per square meter. For wet adhesion tests, once the primers were applied, the concrete blocks were placed in a container with ridges on the bottom allowing water to circulate underneath the blocks. Water, at the test temperature, was added until reaching 1 centimeter below the surface and maintained at that level during the test.
After primer application, the drying time was determined by thumb twist testing according to ASTM 1640. The primer was cured at the designated temperature and humidity for seven days before adhesion testing (ASTM D4541) was carried out. Dollies were glued to the surface and the glue was cured overnight. Dollies were then pulled off using a PAT-adhesion tester. Both the tensile stress at break and the type of failure were recorded. Adhesion tests were carried out in triplicate.
Table 7 summarizes the primer dry times and adhesion to dry and wet concrete. Prototype A gave excellent adhesion to wet and dry concrete, comparable to the commercial product WB-C1 while providing faster dry times.
In general, for waterborne systems, the relative humidity can have a significant impact on drying times and adhesion strength of the coating. High humidity prevents water from evaporating and slows down the cure speed of a coating. Data presented in Table 8 illustrates the characteristics of Prototype A. Even at high humidity, the primer continued to deliver dry speeds of 4 hours. With the increase of humidity to 85%, only 25% loss in cohesive adhesion was observed. The fast drying primer based on Prototype A enables the applicators to apply the floor system in one day by providing sufficient time to apply the topcoat even at 85% humidity.
In addition to the fast drying and excellent adhesion to concrete, another key feature of primers based on Prototype A is the high film build up to about 23.5 mil (600 µm). Results in Table 9 clearly show the retention of drying time and adhesion at higher film builds. Typically in waterborne systems, as the coating thickness increases, it is more difficult for water to evaporate from the coating, especially at low temperature and high humidity. The end result is a longer drying time and entrapped water inside the coating that leads to poor coating appearance and inferior coating properties.
3. Primer overcoatability.
Overcoatability and adhesion strength between primer and topcoat is yet another key requirement to meet. Overcoatability of the primer formulation in Table 5 (Prototype A) was evaluated by cross hatch testing and compared to a solvent-free 2K epoxy primer that used a standard cycloaliphatic amine curing agent (SF primer). Primers were topcoated with a waterborne polyurethane (WB-PU), a white pigmented topcoat based on Prototype A (WB-Epoxy), and a grey pigmented topcoat based on a solvent-free 2K epoxy (SF-Epoxy). The top coats were applied at 400 g/m2 and directly after the primer was dry-to-touch (thumb twist, ASTM D1640). Subsequently, the test was repeated after 1 hour, 2 hours, 1 day, and 1 week after the dry-to-touch time. After the topcoats were cured for 7 days, the systems were subjected to the cross-hatch adhesion test (ISO 2409, rating 1 (excellent adhesion) to 5 (full delamination). The results (Table 10) showed excellent overcoatability. The results were similar to the solvent-free 2K epoxy primer. Longer overcoat windows are the subject of further study.
One-Day Flooring System
Topcoat Performance
We have detailed the properties and excellent performance of the primer formulation based on the new waterborne curing agent Prototype A. In this section, we are focusing on the performance attribute and the key benefits of the flooring system using Prototype A as the topcoat. For this, Prototype A was formulated with diluted liquid epoxy resin in a model topcoat formulation (Table 11).
Basic performance properties of the white paint formulation cured at 10°C/60% RH are summarized in Table 12. Results clearly show the fast dry property of the topcoat formulation. Combining Prototype A in the primer and topcoat will give applicators a solution for a one-day flooring concept.
Intercoat Adhesion Between Primer and Topcoat
As discussed in previous sections, intercoat adhesion between primer and topcoat is critical for the longevity of floor systems. The intercoat adhesion of a floor system formulated with Prototype A in both the primer (Table 5) and the topcoat (Table 11) was evaluated on dry and wet concrete under adverse conditions. The results are summarized in Table 13. The floor system exhibited excellent intercoat adhesion and delivered a walk-on time of less than
24 hours, even on wet concrete conditions. Although using diluted LER in the topcoat lengthened the drying times on wet concrete, it will continue to allow formulators to design a floor system with a return to service after 1 day cure under adverse conditions of low temperature and high humidity.
UV-Resistance of the Floor System
The UV-resistance of topcoats was evaluated by an accelerated UV exposure test. The solvent-free epoxy system chosen is known for its high UV durability feature. Coatings were applied on S36i steel Q-panels at 6 mil (150 µm) wet film thickness and cured for seven days at 23 °C /50% RH. The panels were then placed into a QUV accelerated weathering tester set to a test temperature of 45°C and an irradiance of 0.89 W/m2/nm (UV mode only). Delta E and yellowing index data were obtained over the 250 hour time interval.
Figure 8 exemplifies the very good UV-resistance feature of the new waterborne Prototype A. After 250 hours of UV exposure, a delta E is only one, compared to a delta E of 19 for the SF-epoxy. Prototype A performed marginally better than WB-C1 with a delta E of 2.5.
Conclusions
We have developed a new yellowing-resistant, fast-cure amine building block (Poly HCA) that can be incorporated into a waterborne curing agent. Fundamental studies of Poly HCA shed light on the fast cure property. Poly HCA exhibits an ultra-fast through-cure property that is manifested in high epoxy and amine conversion, thus resulting in high degree of cure. The combination of using IPD with Poly HCA in a new waterborne curing agent, Prototype A, offers a unique solution. The product possesses fast cure speed even under adverse conditions, excellent aesthetics, and outstanding UV resistance. It enables coating formulators to design new floor systems consisting of a primer and a topcoat applied on the same day, and that delivers the fast return to service the next morning. As such, waterborne coating technology continues to meet stringent market requirements both in terms of performance and environmental demands.
Acknowledgement
The authors would like to thank colleagues Mike Oberlander, Daniel Bleumink and Edwin Lijffijt for carrying out the experimental work.
References
1. Richardson F. Pigment and Resin Tech., 1973, 5, 41-43.
2. Zheng, S., Rasing, R. and Bozok, N. Waterborne Symposium, New Orleans, 2018, in press.
3. D.A. Dubowik, F.H. Walker and W.E. Starner, Novel curing agent technology for high solids epoxy coatings, International Waterborne, High Solids, and Powder Coatings Symposium, New Orleans, February 1999.
4. (a) Miller, D.R. and Macosko, C.W. Macromolecules, 1976, 9, 207; b) Miller, D.R., Valles, E.M. and Macosko, C.W. Polym. Eng. Sci., 1979, 19 (4), 272.
5. (a) Lee, W. I., Loos, A. C., Springer, G. S. J. Composite Mat. 1982, 16, 510. Weinmann, D.J., (b) Dangayach, K., Smith, C. Journal of Coatings Technology, 1996, 68 (863), 29-37
6. Xu, L.; Fu, J. H.; Schlup, J. R. J. Am. Chem. Soc. 1994, 116, 2821-2826; Fu, J. H.; Schlup, J. R. J. Appl. Polymer Sci. 1993, 49, 219-227
7. (a) Wegmann, A. Prog. Org. Coatings 1997, 32, 231-239 (b) Walker, F. H. and Schaffer, O.; “Film Formation and Morphology in Two-Component, Ambient-Cured, Waterborne Epoxy Coatings” in Provder, T., Ed. Film Formation, ACS Symposium Series 648, American Chemical Society: Washington D. C., 1996, page 403-417.