Tianqi Liu and Koen Burger, New Product Development, and Technical Service Manager, Cabot Corporation, Boston, MA, USA03.08.19
Aqueous Inkjet Printing Challenges
Over the past twenty years, the printing industry landscape has been redefined by the digital information and communication revolution characterized by a reduction in personal and office printing and increased demand for commercial, signage and packaging printing. As the newest member of the printing technologies, Inkjet has exhibited performance advantages such as high-speed output, flexibility, and easy data processing and customization over traditional printing methods such as offset printing, making it better suited for this transition. In packaging printing, for example, the packages are not only product containers, but also an opportunity to influence customers’ purchasing decisions. The industry need for flexibility around changing the printed packaging for special events, seasonal changes and personalization often result in short print runs where inkjet offers the best cost structure without sacrificing speed and print quality.
There are three basic components in the inkjet printing process: printhead, ink and substrate. From the ink perspective, inkjet can be classified into three categories: solvent, aqueous and UV-curable. Solvent-based inkjet is typically used for applications where durability and scratch resistance are the most important performance requirements. In this process, the solvent “bites” into the substrate surface to anchor the inkjet colorants. The problems with solvent-based inks are solvent handing and work place safety.
UV-curable inkjet can be used on various substrates such as corrugated boards and plastics, however, there are safety and environmental concerns about residual acrylic monomers and photoinitiator migration across the substrate.
Aqueous inkjet ink is the most environmentally safe and user-friendly ink for inkjet printing. It has been adopted for non-plain paper substrates such as glossy offset papers or textiles. In the future expansion of aqueous inkjet in packaging, labels or signage applications, aqueous inkjet must work with even more challenging substrates ranging from very porous substrates such as corrugated boards and canvas, to non-porous and hard-to-adhere-to substrates, such as polyethylene terephthalate (PET) or polyolefins.
There are two major challenges for aqueous inkjet printing on non-paper substrates: liquid handling and fixation of inkjet pigments on the substrate surface. These challenges require that an inkjet receptive coating layer be placed on top of the non-paper substrates to help absorb inkjet liquid and immobilize inkjet pigments. Well-designed inkjet receptive coatings on a wide array of substrates need to work synergistically with an aqueous ink to achieve optimized print performance.
Fumed Metal Oxide Dispersions - Key Component in an Aqueous Inkjet Receptive Coating
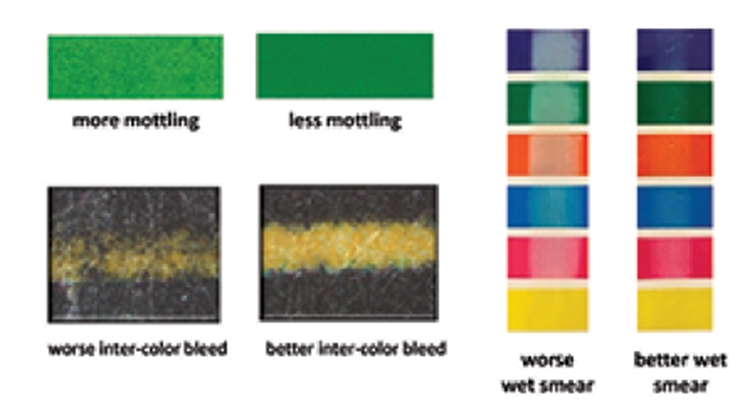
Fumed silica and fumed alumina are high purity inorganic particles that are routinely used for applications in waterborne coating systems for improved hardness, abrasion resistance and rheology control. Unlike calcium carbonate or spherical colloidal silica, fumed silica and fumed alumina feature fractal structures with primary particles arranged into branched chains. The branched aggregate structures are not easy to pack closely, thereby creating intra- and inter-particle voids and channels. These voids or porosity generated by the fractal particles are very important to enable fixation of the pigment in an aqueous inkjet receptive coating. An aqueous ink typically contains around 60-75% water and 20-30% humectants and/or penetrants in liquid form. If the liquid portion of the ink is not absorbed by the receptive coating or not absorbed quickly enough, it can result in image quality issues such as mottling, inter-color bleed and wet smudge (Figure 1).
Although passing the image through a drying zone helps with liquid removal from the printing surface, an inkjet receptive coating is expected to do most of the work because the placement of the drying zone cannot be too close to the printheads or it may lead to ink drying and nozzle clogging issues. Traditional swellable polymer coatings such as starch (or cationic starch) or polyvinyl alcohol-based systems work by a much slower swelling mechanism and hence are not suitable for the high-speed requirement of many printing applications.
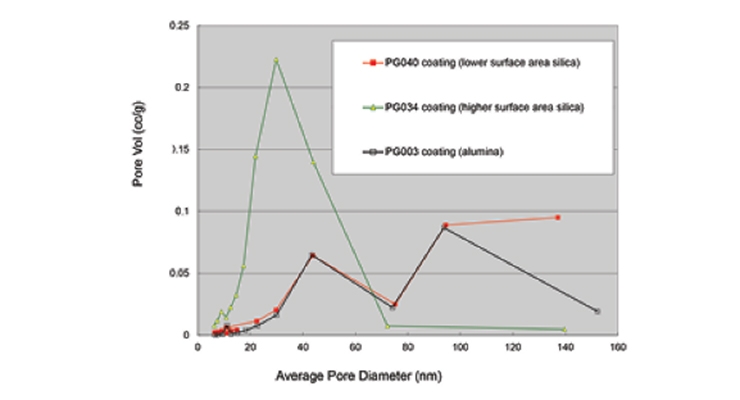
The absorption of liquid is strongly determined by pore volume distribution. Choosing fractal fumed metal oxides with different surface areas or particle chemical compositions (silica versus alumina), can lead to different pore volume versus pore size profiles (Figure 2). The particles in dispersion form are incorporated into a polyvinyl alcohol-based binder system in a 6:1 ratio. Noteworthy is that Cabot’s fumed alumina dispersion (CAB-O-SPERSE® PG003 alumina dispersion) has low pore volume but a roughly bi-modal pore size distribution with a higher fraction of large-sized pores. The low surface area silica shows a similar pore size distribution to that of the PG003 dispersion. Conversely, the higher-surface-area silica shifts the high fraction of pores down to smaller pore diameters, but more volume is held in these smaller pores.
The pores generated in the coatings range from 20 to 150 nm in size. Dynamic fluid absorption in porous media is driven by the capillary pressure, which is inversely proportional to the pore size. Therefore, these nanometer size pores should allow for rapid ink liquid absorption. Typical inkjet colorants, which are 50-180 nm particles, can be partially drawn down into the pores due to capillary forces that act on the liquid. This may be useful for fixation of the colorant particles.
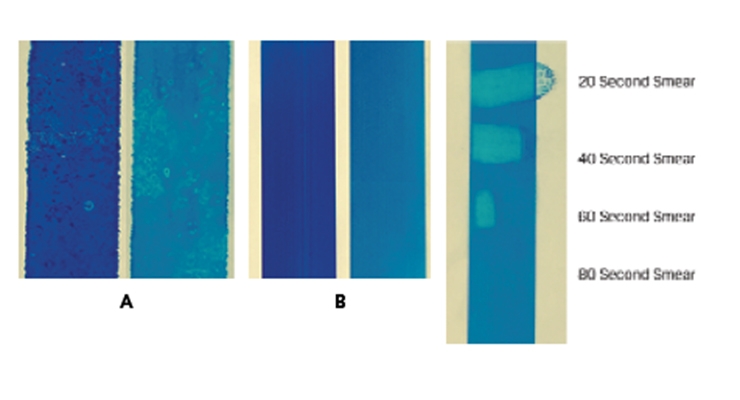
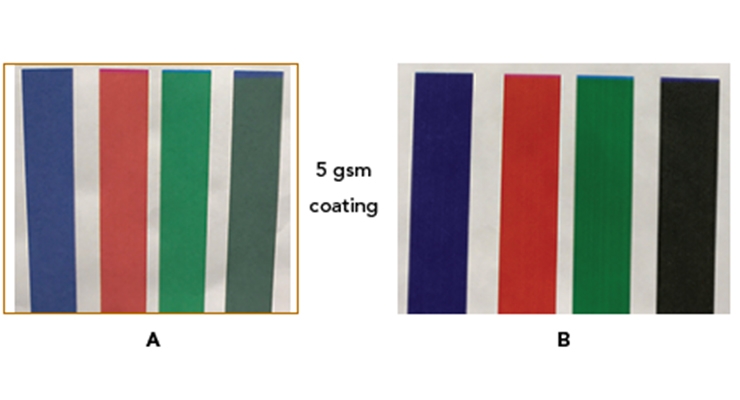
All commercial aqueous inkjet inks use anionically charged colorants, with pigments dominating the market. Without any mechanism of immobilizing the anionic pigments, they will move with the ink liquid on the printing surface, resulting in mottling issues (density variations) and smudge issues on non-porous substrates. Figure 3A shows an aqueous ink printed by Kyocera test bed on a PET substrate: the wetting and puddling issues are obvious, and the print can be easily smudged by fingers even after one week due to the presence of humectants. On very porous substrates such as canvas or some corrugated boards, the pigment particles, due to their nanometer size, will not be retained much on the surface, causing low optical density and feathering issues. As shown in Figure 4A, the color saturation is low on all secondary colors (blue, red and green), and is particularly weak on the composite black.
Untreated fumed silica has an isoelectric point around pH 2.5 and should therefore be anionically charged in typical coating formulations (pH 3-9). The wide range of pKas of surface silanol groups allow un-ionized silanol groups to interact with carboxylate or carboxylic acid groups (usually present on the color pigment surface) by proton transfer or hydrogen bonding. In addition, our proprietary surface treatment can create cationic charges on the silica surface without the use of any cationic dispersants.
Fumed alumina has an isoelectric point around pH 10 and should, therefore, be cationically charged in typical coating formulations (pH 3-9). When an inkjet receptive coating contains fumed alumina, its cationic surface interacts strongly with anionic pigment particles by electrostatic forces, trapping pigment in place and affording high saturation and minimum mottling (Figure 3B and 4B). As mentioned above, there is a benefit to having fumed silica/fumed alumina in a coating to facilitate liquid intake and Figure 3C shows this effect through the finger smudge test on the prints after printing, even without any heating. The print shows quick drying within 80 seconds at room temperature.
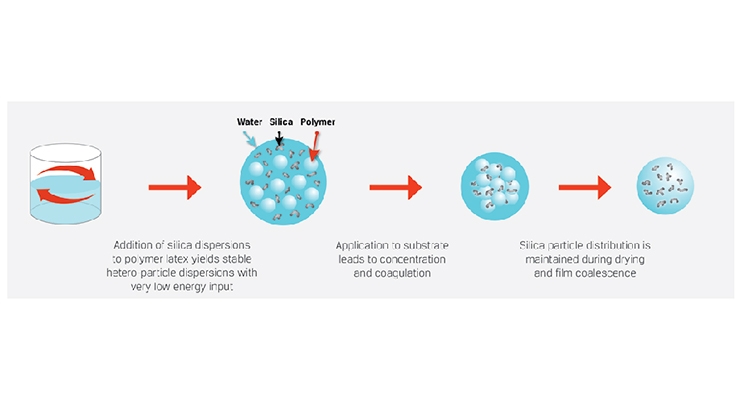
Fumed silica and fumed alumina can sometimes be difficult to work with because they exist as powdery agglomerates that are not easy to disperse into individual aggregates. As manufacturers of coatings continually face challenges associated with meeting stricter environmental standards, the transition from solvent to waterborne has gained momentum in the industry. We produce colloidally stable CAB-O-SPERSE® dispersions to simplify the waterborne coating process, eliminating the need for grinding and dust handling. These fumed silica/fumed alumina dispersions are available in a range of concentrations, particle sizes, surface charges and pH ranges. A schematic illustration of how these dispersions facilitate ease-of-manufacturing in a waterborne coating process is shown in Figure 5.
Dispersions Offer Stability and Flexibility in Waterborne Coatings
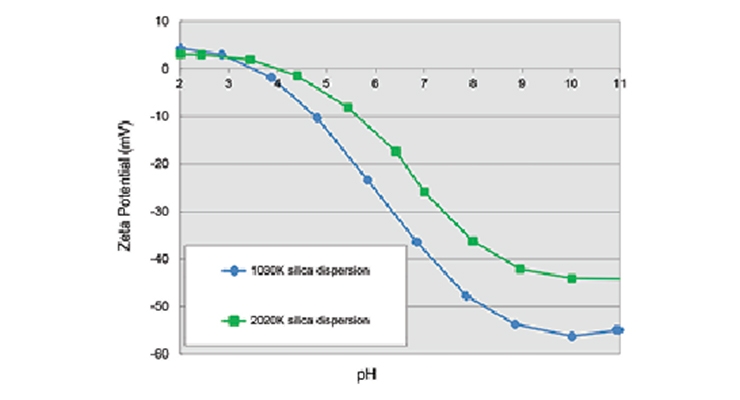
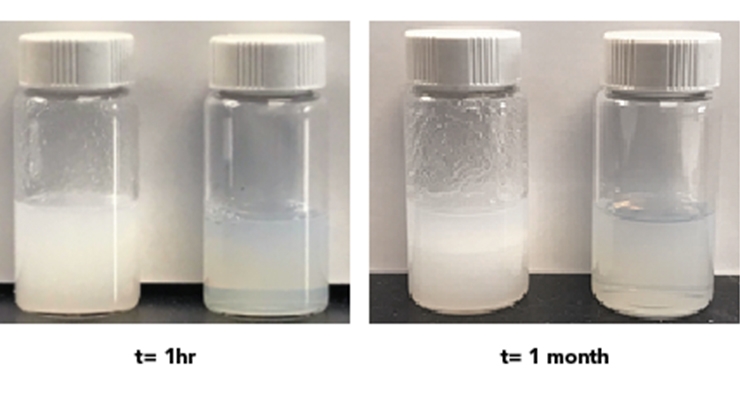
Fumed silica/fumed alumina dispersions lend themselves to coating applications due to the superior degree of dispersion down to the hundreds of nanometer level. The dispersions are very stable as indicated by their high zeta potentials in water (Figure 6). For example, anionic CAB-O-SPERSE® 4012K dispersion offers much better compatibility when mixed at 1:1 weight ratio with a polyvinyl alcohol solution, compared to a surface modified calcium carbonate dispersion, which precipitated out overnight (Figure 7).
The dispersions can maintain their distribution and state of dispersion during addition to latex formulations and during the drying process of a coating, when polymers begin to coalesce and entangle. The optical properties such as clarity or opacity can be tuned by adjusting the coating thickness or by the choice of particles featuring different sizes and surface areas. By virtue of their size, larger particles (e.g., CAB-O-SPERSE® 1030K dispersion) or those with lower specific surface area tend to scatter light more efficiently, generating more opaque coatings. Smaller particles with their higher specific surface area (e.g., the 4012K dispersion) tend to generate more “transparent” coatings. Figure 8 shows the same image under coatings made using the 1030K and 4012K dispersions with the same weight ratio to a polymer binder.
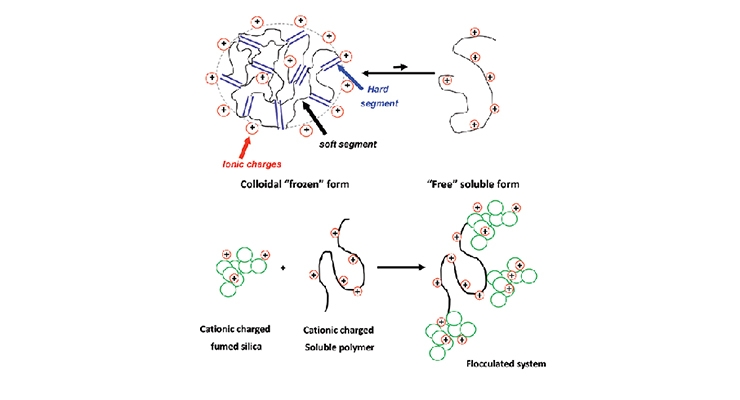
When it comes to cationic coating systems, our fumed alumina dispersions (PG003 and PG008 dispersions) are very robust against cationic polyurethane dispersions or acrylic dispersions. However, the interaction between cationic silica dispersion and cationic polymer latex can be complex. Polyurethane or acrylic dispersions exist in water as multiphase systems, mostly in the form of water-swollen colloidal particles composed of kinetically trapped polymer chains, with some small fraction as free soluble polymers. The partition between particle and soluble forms may shift due to the presence of solvent, surfactant and temperature change. The free chains can bridge silica particle surfaces to form loose agglomerates, leading to phase separation issues as shown in Figure 9. The strategy to eliminate the unfavorable particle resin interactions in such coating systems is to increase the surface cationic charge density, generating a more stable fumed silica system. Comparison between a low density cationic silica vs. a higher density cationic silica together with a cationic polyurethane in 1:1 weight ratio is shown in Figure 10. The higher density treated silica can maintain clarity and stability of the mixture, while low treatment density silica causes the formation of large agglomerates that eventually leads to precipitation or gelation in the system.
Conclusions
As aqueous inkjet continues to take market shares from traditional printing techniques, it must expand its substrates from traditional office paper to a broader spectrum of substrates used for packaging, signage and labels. CAB-O-SPERSE® silica and alumina dispersions can help prime difficult substrates to work with aqueous inkjet inks because of the porosity they generate and their surface charge characteristics. These dispersions enable the formulation of precoats that can greatly enhance optimized image quality such as high color saturation, less mottle and wet smudge resistance. They also offer stability and flexibility in the waterborne coating systems.
Over the past twenty years, the printing industry landscape has been redefined by the digital information and communication revolution characterized by a reduction in personal and office printing and increased demand for commercial, signage and packaging printing. As the newest member of the printing technologies, Inkjet has exhibited performance advantages such as high-speed output, flexibility, and easy data processing and customization over traditional printing methods such as offset printing, making it better suited for this transition. In packaging printing, for example, the packages are not only product containers, but also an opportunity to influence customers’ purchasing decisions. The industry need for flexibility around changing the printed packaging for special events, seasonal changes and personalization often result in short print runs where inkjet offers the best cost structure without sacrificing speed and print quality.
There are three basic components in the inkjet printing process: printhead, ink and substrate. From the ink perspective, inkjet can be classified into three categories: solvent, aqueous and UV-curable. Solvent-based inkjet is typically used for applications where durability and scratch resistance are the most important performance requirements. In this process, the solvent “bites” into the substrate surface to anchor the inkjet colorants. The problems with solvent-based inks are solvent handing and work place safety.
UV-curable inkjet can be used on various substrates such as corrugated boards and plastics, however, there are safety and environmental concerns about residual acrylic monomers and photoinitiator migration across the substrate.
Aqueous inkjet ink is the most environmentally safe and user-friendly ink for inkjet printing. It has been adopted for non-plain paper substrates such as glossy offset papers or textiles. In the future expansion of aqueous inkjet in packaging, labels or signage applications, aqueous inkjet must work with even more challenging substrates ranging from very porous substrates such as corrugated boards and canvas, to non-porous and hard-to-adhere-to substrates, such as polyethylene terephthalate (PET) or polyolefins.
There are two major challenges for aqueous inkjet printing on non-paper substrates: liquid handling and fixation of inkjet pigments on the substrate surface. These challenges require that an inkjet receptive coating layer be placed on top of the non-paper substrates to help absorb inkjet liquid and immobilize inkjet pigments. Well-designed inkjet receptive coatings on a wide array of substrates need to work synergistically with an aqueous ink to achieve optimized print performance.
Fumed Metal Oxide Dispersions - Key Component in an Aqueous Inkjet Receptive Coating
Fumed silica and fumed alumina are high purity inorganic particles that are routinely used for applications in waterborne coating systems for improved hardness, abrasion resistance and rheology control. Unlike calcium carbonate or spherical colloidal silica, fumed silica and fumed alumina feature fractal structures with primary particles arranged into branched chains. The branched aggregate structures are not easy to pack closely, thereby creating intra- and inter-particle voids and channels. These voids or porosity generated by the fractal particles are very important to enable fixation of the pigment in an aqueous inkjet receptive coating. An aqueous ink typically contains around 60-75% water and 20-30% humectants and/or penetrants in liquid form. If the liquid portion of the ink is not absorbed by the receptive coating or not absorbed quickly enough, it can result in image quality issues such as mottling, inter-color bleed and wet smudge (Figure 1).
Although passing the image through a drying zone helps with liquid removal from the printing surface, an inkjet receptive coating is expected to do most of the work because the placement of the drying zone cannot be too close to the printheads or it may lead to ink drying and nozzle clogging issues. Traditional swellable polymer coatings such as starch (or cationic starch) or polyvinyl alcohol-based systems work by a much slower swelling mechanism and hence are not suitable for the high-speed requirement of many printing applications.
The absorption of liquid is strongly determined by pore volume distribution. Choosing fractal fumed metal oxides with different surface areas or particle chemical compositions (silica versus alumina), can lead to different pore volume versus pore size profiles (Figure 2). The particles in dispersion form are incorporated into a polyvinyl alcohol-based binder system in a 6:1 ratio. Noteworthy is that Cabot’s fumed alumina dispersion (CAB-O-SPERSE® PG003 alumina dispersion) has low pore volume but a roughly bi-modal pore size distribution with a higher fraction of large-sized pores. The low surface area silica shows a similar pore size distribution to that of the PG003 dispersion. Conversely, the higher-surface-area silica shifts the high fraction of pores down to smaller pore diameters, but more volume is held in these smaller pores.
The pores generated in the coatings range from 20 to 150 nm in size. Dynamic fluid absorption in porous media is driven by the capillary pressure, which is inversely proportional to the pore size. Therefore, these nanometer size pores should allow for rapid ink liquid absorption. Typical inkjet colorants, which are 50-180 nm particles, can be partially drawn down into the pores due to capillary forces that act on the liquid. This may be useful for fixation of the colorant particles.
All commercial aqueous inkjet inks use anionically charged colorants, with pigments dominating the market. Without any mechanism of immobilizing the anionic pigments, they will move with the ink liquid on the printing surface, resulting in mottling issues (density variations) and smudge issues on non-porous substrates. Figure 3A shows an aqueous ink printed by Kyocera test bed on a PET substrate: the wetting and puddling issues are obvious, and the print can be easily smudged by fingers even after one week due to the presence of humectants. On very porous substrates such as canvas or some corrugated boards, the pigment particles, due to their nanometer size, will not be retained much on the surface, causing low optical density and feathering issues. As shown in Figure 4A, the color saturation is low on all secondary colors (blue, red and green), and is particularly weak on the composite black.
Untreated fumed silica has an isoelectric point around pH 2.5 and should therefore be anionically charged in typical coating formulations (pH 3-9). The wide range of pKas of surface silanol groups allow un-ionized silanol groups to interact with carboxylate or carboxylic acid groups (usually present on the color pigment surface) by proton transfer or hydrogen bonding. In addition, our proprietary surface treatment can create cationic charges on the silica surface without the use of any cationic dispersants.
Fumed alumina has an isoelectric point around pH 10 and should, therefore, be cationically charged in typical coating formulations (pH 3-9). When an inkjet receptive coating contains fumed alumina, its cationic surface interacts strongly with anionic pigment particles by electrostatic forces, trapping pigment in place and affording high saturation and minimum mottling (Figure 3B and 4B). As mentioned above, there is a benefit to having fumed silica/fumed alumina in a coating to facilitate liquid intake and Figure 3C shows this effect through the finger smudge test on the prints after printing, even without any heating. The print shows quick drying within 80 seconds at room temperature.
Fumed silica and fumed alumina can sometimes be difficult to work with because they exist as powdery agglomerates that are not easy to disperse into individual aggregates. As manufacturers of coatings continually face challenges associated with meeting stricter environmental standards, the transition from solvent to waterborne has gained momentum in the industry. We produce colloidally stable CAB-O-SPERSE® dispersions to simplify the waterborne coating process, eliminating the need for grinding and dust handling. These fumed silica/fumed alumina dispersions are available in a range of concentrations, particle sizes, surface charges and pH ranges. A schematic illustration of how these dispersions facilitate ease-of-manufacturing in a waterborne coating process is shown in Figure 5.
Dispersions Offer Stability and Flexibility in Waterborne Coatings
Fumed silica/fumed alumina dispersions lend themselves to coating applications due to the superior degree of dispersion down to the hundreds of nanometer level. The dispersions are very stable as indicated by their high zeta potentials in water (Figure 6). For example, anionic CAB-O-SPERSE® 4012K dispersion offers much better compatibility when mixed at 1:1 weight ratio with a polyvinyl alcohol solution, compared to a surface modified calcium carbonate dispersion, which precipitated out overnight (Figure 7).
The dispersions can maintain their distribution and state of dispersion during addition to latex formulations and during the drying process of a coating, when polymers begin to coalesce and entangle. The optical properties such as clarity or opacity can be tuned by adjusting the coating thickness or by the choice of particles featuring different sizes and surface areas. By virtue of their size, larger particles (e.g., CAB-O-SPERSE® 1030K dispersion) or those with lower specific surface area tend to scatter light more efficiently, generating more opaque coatings. Smaller particles with their higher specific surface area (e.g., the 4012K dispersion) tend to generate more “transparent” coatings. Figure 8 shows the same image under coatings made using the 1030K and 4012K dispersions with the same weight ratio to a polymer binder.
When it comes to cationic coating systems, our fumed alumina dispersions (PG003 and PG008 dispersions) are very robust against cationic polyurethane dispersions or acrylic dispersions. However, the interaction between cationic silica dispersion and cationic polymer latex can be complex. Polyurethane or acrylic dispersions exist in water as multiphase systems, mostly in the form of water-swollen colloidal particles composed of kinetically trapped polymer chains, with some small fraction as free soluble polymers. The partition between particle and soluble forms may shift due to the presence of solvent, surfactant and temperature change. The free chains can bridge silica particle surfaces to form loose agglomerates, leading to phase separation issues as shown in Figure 9. The strategy to eliminate the unfavorable particle resin interactions in such coating systems is to increase the surface cationic charge density, generating a more stable fumed silica system. Comparison between a low density cationic silica vs. a higher density cationic silica together with a cationic polyurethane in 1:1 weight ratio is shown in Figure 10. The higher density treated silica can maintain clarity and stability of the mixture, while low treatment density silica causes the formation of large agglomerates that eventually leads to precipitation or gelation in the system.
Conclusions
As aqueous inkjet continues to take market shares from traditional printing techniques, it must expand its substrates from traditional office paper to a broader spectrum of substrates used for packaging, signage and labels. CAB-O-SPERSE® silica and alumina dispersions can help prime difficult substrates to work with aqueous inkjet inks because of the porosity they generate and their surface charge characteristics. These dispersions enable the formulation of precoats that can greatly enhance optimized image quality such as high color saturation, less mottle and wet smudge resistance. They also offer stability and flexibility in the waterborne coating systems.